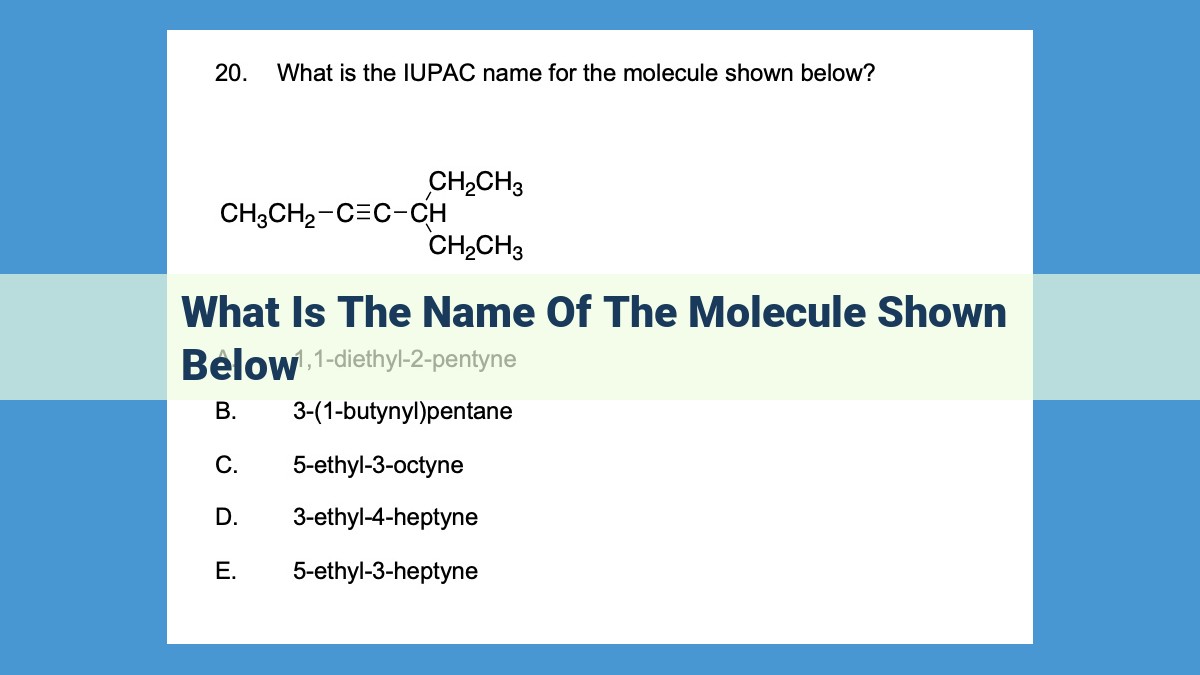
The molecule shown below is called 2,2-dibromo-3-methylbutane. It is an alkane, which is a type of hydrocarbon, with the molecular formula C5H10Br2. It has a parent chain of four carbon atoms (butane) and two bromine atoms and a methyl group attached to the second carbon atom. The IUPAC nomenclature rules are used to name the molecule, ensuring consistency and accuracy in chemical communication.
Deciphering the Molecular Enigma: Unraveling the Secret Behind Molecular Identification
In the realm of science, where knowledge unfolds through the intricacies of molecules, the ability to precisely identify these tiny building blocks is paramount. It’s like unlocking the secrets to a hidden world, unleashing a torrent of insights into their properties, behaviors, and countless applications.
This blog post embarks on a captivating journey to guide you through the art of molecular identification. We’ll unravel the enigma behind deciphering the name of a given molecule, armed with the power of IUPAC nomenclature, the Rosetta Stone of chemistry. So, fasten your seatbelts and let’s dive into the thrilling quest of transforming structural formulas into meaningful names!
Understanding Molecular Nomenclature
- Explain the importance of systematic naming in chemistry.
- Introduce the IUPAC (International Union of Pure and Applied Chemistry) nomenclature rules.
Understanding the Importance of Molecular Nomenclature
In the realm of chemistry, accuracy and precision are paramount. As we delve into the intricate tapestry of molecular structures, a systematic naming system becomes an indispensable tool, enabling scientists to communicate and understand the chemical world with clarity and uniformity. This is where the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules step into the spotlight.
IUPAC Nomenclature: A Guiding Light
Imagine yourself as a chemist exploring a vast library filled with countless molecules. Each molecule is a unique entity, with its own distinct structure and properties. To effectively navigate this vast collection, we need a standardized language to identify and describe each molecule accurately. This is where IUPAC nomenclature comes to our aid.
IUPAC nomenclature is a set of guidelines established by the International Union of Pure and Applied Chemistry. These rules provide a systematic approach to naming organic compounds, ensuring consistency and clarity in scientific communication. By adhering to these rules, chemists can confidently convey the structure and identity of any given molecule.
Unveiling the Essence of Molecules: Determining the Root Name
In the vast realm of chemistry, molecules are the fundamental building blocks of matter. Identifying them accurately is crucial for understanding their properties and applications. The International Union of Pure and Applied Chemistry (IUPAC) has established a standardized system of nomenclature to facilitate this identification.
At the core of this system lies the root name, which reflects the number of carbon atoms in the parent chain, the longest continuous chain of carbon atoms in the molecule. The suffix “-ane”, indicating a saturated hydrocarbon, is appended to the root name.
To determine the root name, we embark on a systematic approach:
-
Count the Carbon Atoms: Determine the number of carbon atoms in the parent chain.
-
Determine the Root Name: Based on the carbon count, assign the corresponding root name:
- 1 carbon: meth-
- 2 carbons: eth-
- 3 carbons: prop-
- 4 carbons: but-
- 5 carbons: pent-
- And so on
For example, a molecule with seven carbon atoms in its parent chain would have a root name of “hept-“.
Identifying Functional Groups
Every molecule consists of a unique story, a symphony of elements harmoniously joined. Within this molecular narrative, functional groups emerge as captivating characters, each with their distinct personality and influence on the molecule’s tale.
To unravel the molecular mystery, scientists have meticulously devised a language, a system of nomenclature, that allows us to decode the secrets hidden within each functional group. By identifying these enigmatic entities, we unlock the key to understanding the molecule’s properties and behavior.
Functional groups are akin to specialized tools, each designed with a specific purpose. They orchestrate the molecule’s reactivity, determining how it interacts with the world around it. Some, like alkyl groups, serve as the molecule’s backbone, providing a foundation of carbon atoms. Others, such as alkenes and alkynes, introduce double and triple bonds, creating sites of high reactivity.
Identifying functional groups is like reading a chemical map, deciphering the clues that reveal the molecule’s true nature. By recognizing these telltale signs, we can piece together the molecule’s story, unraveling its hidden potential and unlocking its secrets.
Numbering the Carbon Chain: A Systematic Approach
In the intricate world of chemistry, assigning numbers to carbon atoms is a crucial step in molecular identification. It’s akin to giving each atom a unique address, ensuring that scientists can communicate precisely about the structure and properties of molecules.
The numbering of the carbon chain follows a set of guidelines established by the International Union of Pure and Applied Chemistry (IUPAC). These guidelines ensure consistency and accuracy in chemical nomenclature, making it easier for scientists around the globe to collaborate and understand each other.
Priority in numbering is given to the functional groups present on the molecule. Functional groups are specific arrangements of atoms that impart unique chemical properties to the molecule. By assigning numbers based on the location of functional groups, we can prioritize the most significant structural features and create a systematic name that accurately reflects the molecule’s identity.
For instance, if a molecule contains both a double bond (alkene) and a hydroxyl group (alcohol), the carbon atom involved in the double bond is assigned a lower number compared to the carbon atom bonded to the hydroxyl group. This prioritization is based on the higher electronegativity and priority of the oxygen atom in the alcohol group.
By following the IUPAC guidelines for numbering the carbon chain, chemists can ensure that the molecular name they assign is consistent, unambiguous, and reflects the true structure of the molecule. This is essential for accurate communication, efficient database management, and the advancement of scientific research.
Including Prefixes and Suffixes to Enhance Nomenclature Precision
To capture the nuances of molecular identity, prefixes and suffixes play a pivotal role in the IUPAC nomenclature system. They provide essential information about the number and nature of substituents adorning the molecular framework.
Prefixes are the gatekeepers of numerical clarity, indicating the quantity of substituents attached to a specific carbon atom. These numerical descriptors precede the substituent name. For instance, “mono-” denotes a single substituent, “di-” signifies two, and “tri-” represents three.
Suffixes, on the other hand, unravel the chemical nature of the substituents. They convey the type of functional group or structural feature associated with the substituent. One common suffix is “-yl,” which designates an alkyl group, a hydrocarbon chain lacking a double or triple bond.
By incorporating prefixes and suffixes into the molecular name, chemists precisely convey the molecular identity, ensuring that no detail is lost in the translation. They serve as the linguistic building blocks that paint an accurate picture of the molecular landscape.
Consider the molecule 2,2-dibromo-3-methylbutane. The prefixes “di-” and “tri-” indicate the presence of two bromine atoms at the second carbon and three hydrogen atoms at the third carbon. The suffix “-yl” denotes the methyl group, a one-carbon alkyl substituent.
Together, these prefixes and suffixes weave an intricate tapestry of information, allowing us to clearly visualize and understand the structural composition of the molecule.
Determining the Name of a Molecule: The Importance of IUPAC Nomenclature
In the realm of chemistry, precisely identifying molecules is crucial for understanding their properties and reactions. This blog post will guide you through the essential steps of naming a molecule based on its structural formula using the International Union of Pure and Applied Chemistry (IUPAC) nomenclature rules.
Understanding Molecular Nomenclature
Systematic naming in chemistry is vital for precise communication and avoids confusion. IUPAC, the governing body for chemical nomenclature, has established a set of rules that provide a standardized and unambiguous way to name molecules.
Identifying the Root Name
The first step is to determine the root name, which indicates the number of carbon atoms in the parent chain. For alkanes (saturated hydrocarbons), the root name is followed by the suffix “-ane.”
Adding Functional Groups
Molecules may contain functional groups, which are specific arrangements of atoms that impart characteristic properties. Identify any functional groups present and use their names as prefixes.
Numbering the Carbon Chain
Numbering the carbon atoms in the parent chain is crucial for correctly identifying substituents. Functional groups have priority in determining the correct numbering.
Including Prefixes and Suffixes
Prefixes indicate the number of substituents (e.g., mono-, di-, tri-), while suffixes indicate their type (e.g., -yl for alkyl groups).
Example: 2,2-Dibromo-3-Methylbutane
Let’s apply these rules to name the molecule 2,2-Dibromo-3-Methylbutane:
- Root name: “But” (4 carbon atoms in the parent chain)
- Functional groups: Two bromo groups (prefixes: di-) and one methyl group (prefix: 3-Methyl-)
- Numbering: The bromo groups are on carbon atoms 2 and 2, and the methyl group is on carbon atom 3.
Therefore, the complete name of the molecule is 2,2-Dibromo-3-Methylbutane.
In conclusion, using IUPAC nomenclature rules allows us to accurately identify and name molecules, facilitating scientific communication and our understanding of their properties.
Demystifying Molecular Nomenclature: A Guide to Identifying Molecules
In the realm of chemistry, unraveling the identity of molecules is paramount. Through the systematic naming of these enigmatic entities, we can unlock their secrets and understand their multifaceted roles in our world. In this blog post, we embark on a guided tour of molecular identification, deciphering the language that unveils the names of molecules based on their intricate structures.
Understanding the Nomenclature Enigma
The International Union of Pure and Applied Chemistry (IUPAC) has established a set of universal guidelines for naming molecules, ensuring clarity and consistency in scientific communication. These rules serve as the roadmap for our journey into molecular nomenclature.
Laying the Foundation: Root Names and Functional Groups
The backbone of every molecule’s name lies in its root name, which reflects the number of carbon atoms in its parent chain. Functional groups, chemical entities that impart distinctive properties, further modify the root name. Alkyl groups, alkenes, and alkynes are a few examples of these molecular embellishments.
Numbering the Carbon Chain: A Strategic Approach
Assigning numbers to carbon atoms within the chain is a crucial step. Priority is given to functional groups, as they dictate the correct numbering sequence. This strategic approach ensures that the molecule’s name accurately reflects its structural intricacies.
Prefixes and Suffixes: Completing the Puzzle
Prefixes indicate the number of substituents adorning the molecule, while suffixes denote their nature. These linguistic tools provide the finishing touches, completing the molecular name like pieces of a puzzle.
A Real-Life Case Study: 2,2-Dibromo-3-Methylbutane
To illustrate the practical application of IUPAC nomenclature rules, let’s delve into the naming of a specific molecule: 2,2-Dibromo-3-methylbutane. Tracing through the steps outlined above, we unravel its identity, breaking down the structural formula into a comprehensible name.
Additional Insights: Unveiling the Molecule’s Secrets
Beyond the name, additional information sheds light on the molecule’s characteristics. The molecular formula and molecular weight provide quantitative insights into its composition and mass. The structural formula depicts its atomic arrangement, revealing the three-dimensional architecture of the molecule. The CAS Registry Number serves as a unique identifier in chemical databases, facilitating further exploration.
Epilogue: The Power of Molecular Identification
Accurately identifying molecules is not merely an academic exercise. It empowers us to unravel their properties, predict their reactivity, and harness their potential in various applications. From drug discovery to materials science, molecular identification serves as a gateway to unlocking the boundless possibilities of chemistry.