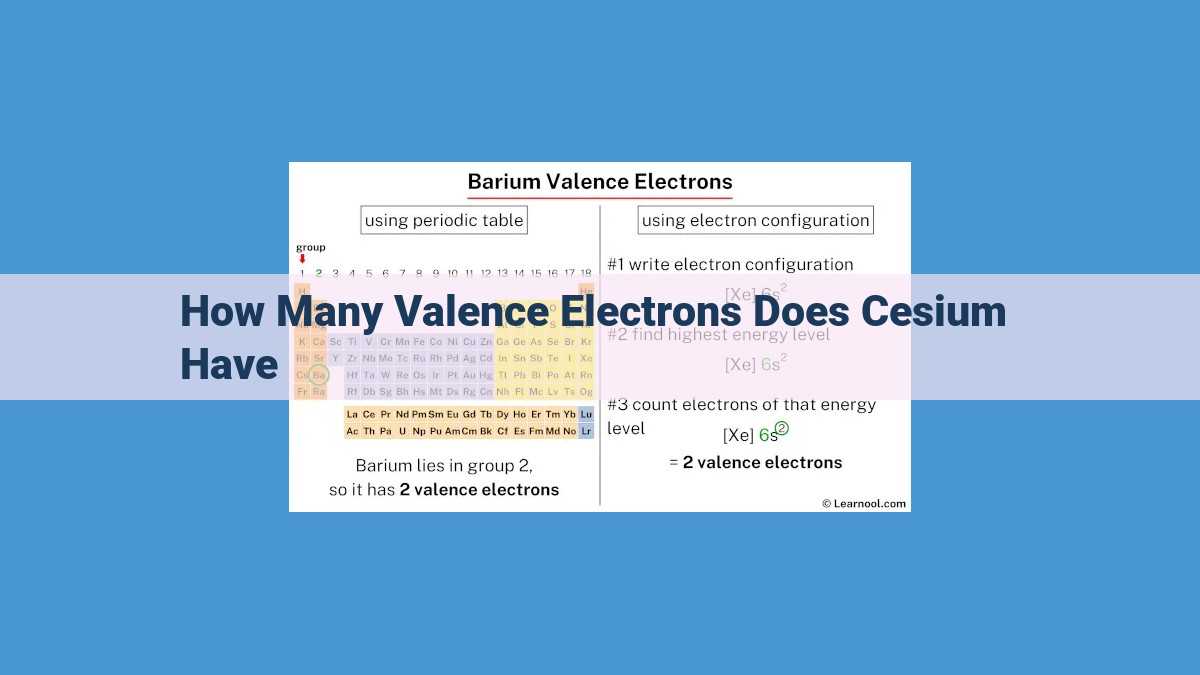
Cesium, an alkali metal, has a single valence electron in its outermost energy level. This lone electron plays a crucial role in determining cesium’s high reactivity and characteristic properties. The number of valence electrons in an element is directly related to its atomic number and electron configuration, which are determined by the Aufbau principle and Hund’s rule.
Valence Electrons: The Key to Chemical Reactions
In the realm of chemistry, the concept of valence electrons is paramount in understanding chemical reactions and predicting elemental behavior. These are the electrons that reside in the outermost energy level of an atom, playing a pivotal role in the formation and breaking of chemical bonds.
Imagine valence electrons as the social butterflies of the atomic world, eagerly seeking interactions with other atoms. This inherent sociability stems from their energetic state, which makes them unstable and prone to gaining or losing electrons to achieve stability.
The number of valence electrons an atom possesses dictates its chemical reactivity. The more valence electrons available for interactions, the more reactive the atom. This delicate dance between valence electrons and chemical reactivity is crucial for understanding why certain elements readily form bonds while others remain aloof.
Moreover, valence electrons have a fascinating relationship with atomic orbitals. Think of atomic orbitals as the designated parking spaces for electrons within an atom. Each valence electron occupies a specific atomic orbital, dictating its quantum state and influencing the atom’s overall ionization energy.
The Enigmatic Element: Cesium and Its Unique Properties
In the realm of chemistry, valence electrons hold the key to understanding the behavior and properties of elements. Cesium, an alkali metal with a rich history and intriguing characteristics, serves as an exemplary case study in exploring the significance of valence electrons.
Cesium is an element that captures attention with its extreme softness and high reactivity. Its silvery-white appearance belies its flammability, making it a substance that demands both respect and caution in handling. This remarkable reactivity stems from the single valence electron that Cesium possesses.
The classification of Cesium as an alkali metal, or Group 1 element, is no coincidence. Alkali metals are renowned for their ability to readily give up their outermost electron, a trait that Cesium exhibits with unmatched enthusiasm. This single valence electron makes Cesium an exceptionally effective reducing agent, capable of transferring electrons to other elements and molecules with ease.
Cesium’s unique properties have earned it a niche in various applications. Its high reactivity has made it essential in the production of photocells and electron tubes. Its low melting point and high thermal conductivity have also found use in the field of cryogenics. Additionally, Cesium’s atomic clock has revolutionized timekeeping, providing unparalleled accuracy in scientific research and everyday life.
Atomic Number and Electron Configuration: Unveiling Cesium’s Inner Workings
Delving into the Heart of an Atom: The Atomic Number
Imagine an atom as a bustling city, with protons zipping around the nucleus like tiny commuters and electrons flitting about like vibrant street performers. The atomic number, a fundamental characteristic of every element, tells us the precise number of protons residing in that atom’s nucleus, dictating the element’s identity. For cesium, our star performer, this number is 55, indicating its unique place as the 55th element in the periodic table.
Unveiling the Electron Configuration: The Aufbau Principle and Hund’s Rule
Now, let’s step outside the nucleus and explore the electron configuration of cesium, a blueprint revealing the arrangement of electrons in its energy levels. As we delve into this quantum world, we’ll rely on two guiding principles: the Aufbau principle and Hund’s rule.
The Aufbau principle, like a meticulous construction worker, dictates that electrons occupy the lowest energy levels first, gradually building up the electronic structure. In cesium, electrons progressively fill the 1s, 2s, and 2p orbitals.
Next, Hund’s rule, the playful rebel of quantum physics, ensures that electrons avoid crowding together. Instead, they prefer to occupy separate orbitals within the same energy level, adopting a “social distancing” approach with their spins aligned in parallel, like tiny magnets with matching poles.
Peeling Back the Layers: Cesium’s Electron Configuration
Guided by these principles, we can peel back the layers of cesium’s electron configuration:
- Energy Level 1 (n=1): The innermost energy level, housing the 1s orbital, is occupied by 2 electrons.
- Energy Level 2 (n=2): The second energy level comprises the 2s and 2p orbitals. The 2s orbital accommodates 2 electrons, while the 2p orbital hosts 6 electrons, filling all three of its suborbitals.
- Energy Level 3 (n=3): This outermost energy level contains the 3s, 3p, and 3d orbitals. The 3s orbital is where cesium’s single valence electron resides, giving it its distinctive chemical properties.
Thus, cesium’s electron configuration is 1s² 2s² 2p⁶ 3s¹ 3p⁶ 3d¹⁰ 4s¹ 4p⁶ 4d¹⁰ 4f¹⁴ 5s² 5p⁶ 5d¹⁰ 6s¹. This intricate arrangement of electrons shapes cesium’s unique identity and governs its behavior in the chemical realm.
Valence Electrons in Cesium: A Puzzle Piece for Understanding Elemental Behavior
Embark on a journey into the enigmatic world of valence electrons, the unsung heroes that hold the key to understanding chemical reactions and the unique characteristics of elements like cesium.
Unveiling Cesium’s Secrets:
Cesium, an alluring alkali metal, resides in Group 1 of the periodic table. Its allure lies in its captivating properties: softness, breathtaking reactivity, and an affinity for catching fire. But what sets this element apart is its electronic structure, particularly its valence electrons.
The Power of One:
Cesium boasts a unique electron configuration, with a single electron perched in its outermost energy level. This solitary valence electron becomes the driving force behind cesium’s remarkable reactivity. It’s like an eager explorer, ready to leap into the unknown and form new chemical bonds.
The Catalyst for Reactivity:
The presence of this lone valence electron makes cesium highly susceptible to ionization. This means it willingly gives up its electron to other atoms, forming positive ions. This exceptional readiness to share its electron explains cesium’s high reactivity. It’s like an eager social butterfly, always seeking companionship through chemical bonding.
Cesium’s solitary valence electron serves as a crucial puzzle piece in deciphering its chemical behavior. It provides a glimpse into the intricate dance of electrons, shaping the element’s properties and influencing its interactions with the world around it. Understanding valence electrons is essential for unravelling the mysteries of chemistry and appreciating the captivating diversity of elements like cesium.