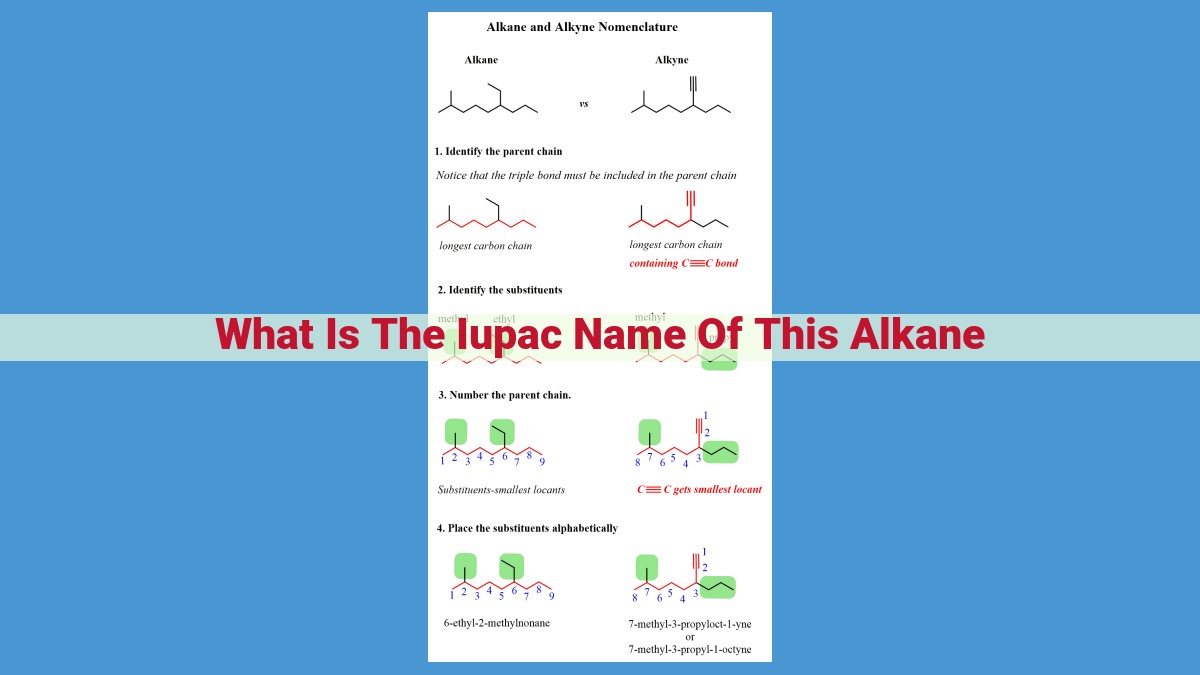
The IUPAC name of an alkane is derived systematically to provide precise and unambiguous identification. It involves identifying the parent chain (the longest carbon chain), numbering its carbon atoms, and selecting the appropriate prefixes and suffixes based on the number and position of substituents (other carbon chains or groups) attached to it. The name consists of the prefix indicating the number of carbon atoms in the parent chain, followed by the suffix “-ane” to signify its alkane nature, and any substituents are named as prefixes with their location indicated by numbers. This standardized nomenclature ensures consistent and clear communication among chemists.
Unraveling the Secrets of Alkanes: A Guide to IUPAC Nomenclature
If you’re immersed in the fascinating world of chemistry, you’ve likely encountered alkanes, the simplest and most fundamental family of organic compounds. To ensure clarity and consistency in naming these compounds, the International Union of Pure and Applied Chemistry (IUPAC) has established a standardized system of nomenclature.
Alkanes are saturated hydrocarbons, meaning their carbon atoms are bonded together by single bonds only. They form the building blocks of a vast array of chemical compounds and play a crucial role in our daily lives. To effectively communicate and understand the intricate structures of alkanes, it’s essential to grasp the intricacies of IUPAC nomenclature.
IUPAC’s Role in Standardization
IUPAC’s primary mission is to standardize chemical nomenclature, ensuring that scientists worldwide can accurately convey complex chemical structures in a common language. By establishing clear rules and guidelines, IUPAC promotes effective scientific communication and facilitates collaboration among researchers.
Identifying the Parent Chain:
- Explain the concept of the parent chain and its importance in alkane naming.
Identifying the Parent Chain: The Backbone of Alkanes
In the realm of organic chemistry, alkanes hold a foundational position, serving as the simplest class of hydrocarbons. To navigate the world of alkanes, a standardized system of nomenclature known as IUPAC (International Union of Pure and Applied Chemistry) nomenclature is essential. This system ensures clear and unambiguous communication among chemists.
The cornerstone of IUPAC alkane nomenclature lies in identifying the parent chain. The parent chain is the longest continuous chain of carbon atoms in the molecule. It forms the backbone upon which the rest of the molecule is built.
Recognizing the parent chain is crucial because it determines the root name of the alkane. The number of carbon atoms in the parent chain dictates the prefix of the alkane name, while the suffix -ane indicates its status as an alkane.
For instance, consider the alkane with the molecular formula CH3CH2CH2CH2CH3. By identifying the longest continuous chain of carbon atoms, we establish that the parent chain has five carbons. This corresponds to the prefix pent-. Since it’s an alkane, the suffix is -ane. Thus, the IUPAC name of this alkane becomes pentane.
Determining the parent chain is not always straightforward. In certain cases, multiple chains with the same length may be present. When faced with such ambiguity, chemists adopt specific rules to ensure consistent naming. These rules prioritize certain types of carbon-carbon bonds, ensuring the selection of the most representative chain as the parent chain.
By understanding the concept of the parent chain and mastering the art of identifying it, we establish a solid foundation for understanding IUPAC alkane nomenclature. It’s a crucial step that empowers us to navigate the complexities of organic chemistry with precision and clarity.
Understanding Substituents: The Building Blocks of Complex Organic Compounds
In the realm of organic chemistry, where molecules dance and form intricate arrangements, substituents play a crucial role in determining the structure and properties of compounds. These versatile chemical entities, like tiny building blocks, attach themselves to the backbone of molecules, modifying their characteristics and giving rise to a vast array of organic substances.
Substituents can be broadly classified into three main types:
- Alkyl groups: Derived from alkanes, these substituents consist of carbon and hydrogen atoms bonded together in a straight chain or branched structure. They are named according to the number of carbon atoms they contain, such as methyl (CH3), ethyl (C2H5), and propyl (C3H7).
- Functional groups: These are specific groups of atoms that impart characteristic chemical properties to molecules. Common functional groups include hydroxyl (-OH), carbonyl (C=O), and amine (-NH2). They determine the reactivity and functionality of organic compounds, allowing them to undergo specific reactions and interact with other molecules.
- Heteroatoms: These are non-carbon atoms that can be incorporated into organic molecules. They include oxygen, nitrogen, sulfur, and halogens. Heteroatoms can modify the polarity, solubility, and reactivity of compounds, further expanding the diversity and properties of organic substances.
The presence of substituents on a molecule can drastically alter its physical and chemical behavior. They can affect boiling points, solubility, acidity, and reactivity. For example, the addition of a hydroxyl group makes a compound more polar and water-soluble, while the introduction of a halogen atom can increase a compound’s reactivity.
By understanding the types and properties of substituents, chemists can predict the behavior of organic compounds and design molecules with specific functionalities for various applications. From pharmaceuticals and fragrances to plastics and fuels, substituents play an essential role in shaping the molecular world around us.
Numbering Carbon Atoms: The Key to Unraveling the Structure of Alkanes
When it comes to naming alkanes, the backbone of organic chemistry, identifying the parent chain is crucial. But how do we determine which carbon atoms belong to this crucial chain? The answer lies in the systematic approach of numbering carbon atoms.
Imagine the alkane molecule as a tiny train with carbon atoms as its carriages. Our goal is to assign numbers to these carriages in a way that ensures the lowest possible set of numbers for the substituents attached to them.
We start by selecting the longest continuous chain of carbon atoms, which becomes our parent chain. Next, we begin numbering the carbon atoms in this chain from one end, always choosing the direction that gives the lowest numbers to the substituents.
If we encounter any branches or side chains along the way, we assign them a number based on the carbon atom they are attached to. For example, if a methyl group (CH3) is attached to the third carbon of the parent chain, we would designate it as “3-methyl.”
By following this systematic approach, we can uniquely identify each carbon atom in the alkane molecule, providing the foundation for its IUPAC name.
Prefixes and Suffixes: The Key to Understanding Alkane Names
Just like people have first names and last names, alkanes have prefixes and suffixes to identify them clearly. Prefixes tell us the number of carbon atoms in the parent chain, while suffixes indicate the alkane family. This naming system is standardized by the International Union of Pure and Applied Chemistry (IUPAC), the governing body for chemical nomenclature.
Prefixes: Counting the Carbon Atoms
Prefixes are simple and straightforward:
- Meth- for 1 carbon atom
- Eth- for 2 carbon atoms
- Prop- for 3 carbon atoms
- But- for 4 carbon atoms
- Pent- for 5 carbon atoms
So, for example, ethane has a two-carbon parent chain (eth-), while butane has a four-carbon parent chain (but-).
Suffixes: Identifying the Alkane Family
Suffixes are equally essential. They tell us what type of alkane we’re dealing with:
- -ane for alkanes (single bonds between carbon atoms)
- -ene for alkenes (double bond between carbon atoms)
- -yne for alkynes (triple bond between carbon atoms)
By combining prefixes and suffixes, we can build the complete IUPAC name for an alkane. For instance, butane is an alkane with a four-carbon parent chain (but-), and its suffix (-ane) indicates that it only has single bonds between its carbon atoms.
Navigating the World of Alkanes: A Comprehensive Guide to IUPAC Nomenclature
Embark on an enlightening journey into the realm of alkanes and unravel the intricacies of their nomenclature using the standardized guidelines established by the International Union of Pure and Applied Chemistry (IUPAC).
Identifying the Parent Chain: The Foundation of Nomenclature
The parent chain serves as the backbone of an alkane. It is the longest continuous chain of carbon atoms within the molecule. Identifying the parent chain is crucial as it determines the base name of the compound.
Understanding Substituents: The Accompanying Units
Alkanes can have branches or side chains attached to their parent chain, known as substituents. Substituents can be alkyl groups (groups derived from alkanes) or other functional groups. Their presence and position along the parent chain influence the compound’s name.
Numbering Carbon Atoms: A Systematic Approach
To assign names accurately, each carbon atom in the parent chain must be numbered systematically. Start numbering from the end of the chain closest to a substituent. If there are multiple substituents, choose the one with the lowest number.
Prefixes and Suffixes: The Language of Alkanes
The number of carbon atoms in the parent chain is denoted by prefixes, such as “meth-” for one carbon, “eth-” for two carbons, and so on. The suffix “-ane” indicates that the compound is an alkane.
Steps for IUPAC Alkane Nomenclature: A Step-by-Step Guide
- Identify the parent chain: Locate the longest continuous chain of carbon atoms.
- Identify and number substituents: Determine the type and position of any substituents on the parent chain.
- Assign prefixes and suffixes: Use prefixes to indicate the number of carbons in the parent chain and “-ane” as the suffix.
- Assemble the name: Combine the prefix, substituent names (in alphabetical order), and parent chain name to form the complete IUPAC name.
For example, the IUPAC name for the alkane with the formula CH3-CH(CH3)-CH2-CH2-CH3 is 2-methylpentane.
Practical Examples of IUPAC Nomenclature: Putting Theory into Practice
Exploring real-world examples solidifies our understanding. Here are a few:
- Butane: CH3-CH2-CH2-CH3 (parent chain: 4 carbons; no substituents)
- 2-methylpropane: (CH3)3-CH (parent chain: 3 carbons; 1 methyl substituent at carbon 2)
- 3-ethylhexane: CH3-CH2-CH(CH2CH3)-CH2-CH2-CH3 (parent chain: 6 carbons; 1 ethyl substituent at carbon 3)
Importance of IUPAC Alkane Nomenclature: A Unified Language
Standardized nomenclature ensures clear and consistent communication among chemists. It facilitates accurate identification, synthesis, and characterization of alkanes, laying the foundation for scientific progress and innovation.
By mastering IUPAC alkane nomenclature, you gain a powerful tool for navigating the diverse world of organic chemistry. Embrace the journey and unlock the secrets of these fundamental hydrocarbons!
Practical Examples of IUPAC Nomenclature:
- Include examples of IUPAC-named alkanes and explain their derivation.
Practical Examples of IUPAC Nomenclature
To illustrate the practical application of IUPAC nomenclature, let’s delve into a few real-world examples:
Butane:
This alkane consists of four carbon atoms arranged in a straight chain. The parent chain is but**, and since the compound contains four carbons, the _ane_ suffix is used. Therefore, the IUPAC name of butane is butane.
2-Methylpropane:
This branched alkane has a three-carbon parent chain and a single methyl group as a substituent on the second carbon. The substituent is named _methyl_ and is positioned using the _2- prefix. Thus, the IUPAC name becomes 2-methylpropane.
3-Ethylhexane:
Here, we have a six-carbon parent chain with an ethyl substituent on the third carbon. The _3- prefix indicates the position of the substituent, and _hexane_ denotes the six-carbon chain. Therefore, the IUPAC name for this compound is _3-ethylhexane_.
2,2-Dimethylbutane:
In this case, there are two methyl substituents attached to the second carbon of the parent chain. The _2,2- prefixes indicate the presence of multiple identical substituents on the same carbon. Thus, the IUPAC name is _2,2-dimethylbutane_.
These examples demonstrate the systematic approach of IUPAC nomenclature, ensuring clear and consistent naming of alkanes. This standardized system facilitates effective communication and comprehension in the field of chemistry.
The Importance of IUPAC Alkane Nomenclature: Facilitating Clear Communication in Chemistry
When it comes to naming chemical compounds, consistency and precision are paramount. Standardization in nomenclature ensures that chemists, researchers, and students worldwide can communicate effectively and avoid misunderstandings. This is where the International Union of Pure and Applied Chemistry (IUPAC) plays a crucial role.
IUPAC’s alkane nomenclature provides a systematic set of rules for naming alkanes, the simplest class of hydrocarbons. These rules establish a common language, allowing chemists to describe and identify alkanes accurately and unambiguously.
You might wonder, why is standardized nomenclature so important? Consider this: if every chemist used their own unique naming system, the chaos would make it nearly impossible to collaborate, share research, or understand scientific literature. It would be like trying to decipher a secret code, wasting valuable time and effort.
By adopting IUPAC alkane nomenclature, scientists ensure that every compound has a specific, recognizable name. This enables them to:
- Clearly identify and discuss chemical compounds in research papers, presentations, and textbooks.
- Facilitate communication among chemists of different languages and backgrounds.
- Avoid confusion and ambiguity when describing and referencing alkanes.
- Create databases and retrieve information about alkanes efficiently and accurately.
- Promote collaboration and the exchange of knowledge in the field of chemistry.
The benefits of IUPAC alkane nomenclature extend beyond the realm of chemistry. Standardization in scientific terminology is essential for collaboration and progress in any field. It allows researchers to build upon existing knowledge, share discoveries, and advance human understanding. Without a standardized nomenclature system, the exchange of scientific information would be severely hampered, hindering innovation and discovery.
Therefore, embracing IUPAC alkane nomenclature is not merely a matter of following rules. It is a testament to the importance of collaboration, precision, and the pursuit of scientific excellence. By adhering to these standardized guidelines, we foster a shared language that empowers chemists to communicate effectively, advance knowledge, and shape the future of scientific discovery.