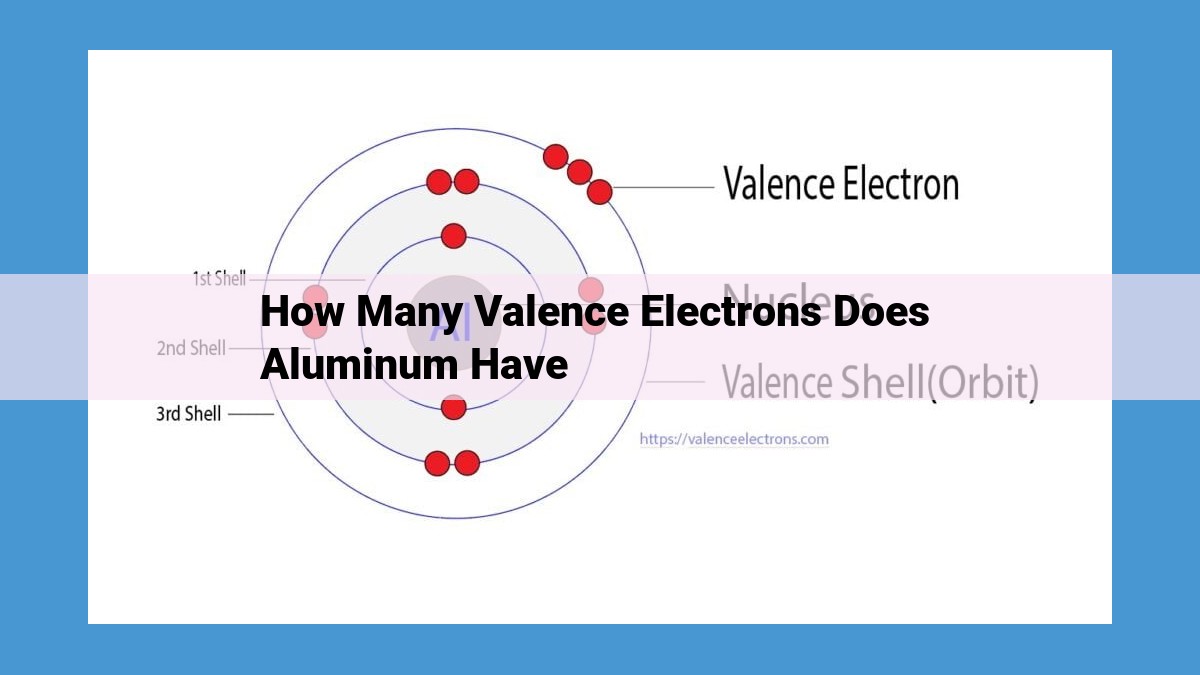
Aluminum, with an atomic number of 13, possesses three valence electrons. These electrons reside in the outermost shell (3p orbital) and play a crucial role in the chemical behavior and bonding properties of aluminum. Valence electrons participate in forming covalent bonds and molecular orbitals, determining the reactivity and bonding characteristics of aluminum. Understanding valence electrons is essential for comprehending the electronic structure of aluminum and its chemical interactions.
Valence Electrons: An Overview
- Definition and role of valence electrons in chemical reactions
- Related concepts: atomic orbitals and electron configuration
Valence Electrons: The Key Players in Chemical Reactions
Imagine atoms as miniature solar systems, with electrons orbiting the nucleus like planets. Among these electrons, there’s a special group called valence electrons. They’re the ones that venture farthest from the nucleus, and they play a crucial role in determining how atoms interact with each other.
Understanding Valence Electrons and Their Impact
Valence electrons are like the dating pool of the atomic world. They’re the electrons that participate in chemical reactions, forming bonds with other atoms. The number of valence electrons an atom has influences its reactivity. Atoms with more valence electrons are more likely to form bonds and react, while those with fewer valence electrons are more stable and less reactive.
Determining Aluminum’s Valence Electrons
Let’s take aluminum as an example. Its atomic number is 13, which means it has 13 electrons. These electrons are arranged in different energy levels, or atomic orbitals, like shells around the nucleus. The outermost shell, or valence shell, can hold up to 8 electrons.
In aluminum’s case, the electron configuration is 1s²2s²2p⁶3s²3p¹. This means that it has 2 electrons in the first shell (1s), 2 in the second shell (2s), 6 in the third shell (2p), and 1 in the fourth and outermost shell (3p). The valence electrons, therefore, are the ones in the valence shell, which in the case of aluminum is just 1.
Determining Aluminum’s Valence Electrons
In the realm of chemistry, every element possesses unique characteristics that dictate its behavior and interactions. One fundamental property of an element is its valence electrons, which play a pivotal role in chemical reactions. To understand these crucial electrons, let’s embark on a journey to determine the valence electrons of the versatile metal, Aluminum.
Aluminum’s atomic number, 13, reveals that it has 13 electrons orbiting its nucleus. These electrons occupy specific energy levels, with the outermost level being the most influential in chemical bonding. The electron configuration of Aluminum, 1s²2s²2p⁶3s²3p¹, provides a detailed roadmap of how its electrons are distributed.
The outermost energy level, denoted by 3p, contains one electron. This lone electron, perched on the brink of Aluminum’s electronic structure, is the element’s valence electron. Valence electrons are like the extroverted diplomats of the atom, eager to venture beyond their atomic boundaries and interact with other atoms.
In the dynamic world of chemical reactions, valence electrons are the decisive players. They determine the element’s reactivity, its ability to bond with other elements, and the types of bonds it can form. Aluminum’s single valence electron grants it a keen affinity for other elements, making it a versatile participant in a wide range of chemical processes.
The Significance of Valence Electrons in Aluminum’s Reactivity and Chemical Bonding
Delving into the Realm of Valence Electrons
Every atom, the fundamental building block of matter, possesses electrons that orbit its nucleus. Among these electrons, valence electrons hold a unique significance, as they determine an atom’s chemical behavior. Valence electrons occupy the outermost shell of an atom, directly influencing its ability to interact with other atoms.
Unveiling Aluminum’s Valence Electron Count
Aluminum, a versatile and ubiquitous metal, showcases three valence electrons. Its atomic number, which represents the number of protons in its nucleus, is 13. This atomic number dictates that aluminum has 13 electrons in total, with three of them occupying the 3p subshell of the outermost energy level.
Valence Electrons: Gatekeepers of Reactivity
The presence of these three valence electrons profoundly impacts aluminum’s reactivity. Valence electrons act as the emissaries of chemical reactions, enabling aluminum to interact with other elements. Aluminum’s reactivity stems from its low ionization energy, which facilitates the detachment of these valence electrons. This willingness to share electrons makes aluminum an eager participant in chemical bonding.
Valence Electrons: Architects of Bonding
In the realm of chemical bonding, valence electrons play a pivotal role. They determine the type and strength of bonds formed between atoms. Aluminum, with its three valence electrons, exhibits a proclivity for covalent bonding. Covalent bonds arise when atoms share valence electrons, resulting in the formation of stable molecules. In aluminum’s case, these valence electrons engage in covalent bonding, leading to the creation of aluminum-containing molecules that exhibit unique properties.
Molecular Orbital Theory and Valence Electrons
As we delve into the fascinating world of chemistry, we encounter the concept of molecular orbitals—a crucial aspect of understanding the electronic structure and bonding behaviors of molecules. Molecular orbitals are mathematical functions that describe the possible wave-like behavior of electrons within a molecule.
These orbitals are formed by the superposition of individual atomic orbitals belonging to the valence electrons of the atoms involved in the molecule. Valence electrons are those electrons residing in the outermost energy shell of an atom, playing a pivotal role in chemical reactions and bonding.
When atoms combine to form a molecule, their valence electrons interact, overlapping to form molecular orbitals. These molecular orbitals can be classified into different types, such as bonding orbitals (which stabilize the molecule) and antibonding orbitals (which weaken the molecule).
The distribution of valence electrons in molecular orbitals dictates the bonding properties of the molecule. Molecules with a higher number of stabilizing bonding orbitals will be more stable and less reactive, while those with more antibonding orbitals will be less stable and more reactive.
Comprehending the concept of molecular orbitals is paramount for deciphering the intricate chemical behaviors observed in the world around us. It provides a theoretical framework for rationalizing the reactivity, stability, and electronic properties of molecules, empowering scientists to design and synthesize new materials with tailored properties.