Anaerobic respiration, occurring in the cytoplasm and lacking an electron transport chain, produces a limited ATP yield (2 ATP) through substrate-level phosphorylation. In contrast, aerobic respiration, occurring in mitochondria with a complex electron transport chain, generates a significantly higher ATP yield (36-38 ATP) through oxidative phosphorylation. This enhanced energy efficiency makes aerobic respiration the more favorable energy-yielding process in most organisms.
Anaerobic and Aerobic Respiration: Unraveling the Energy Dance
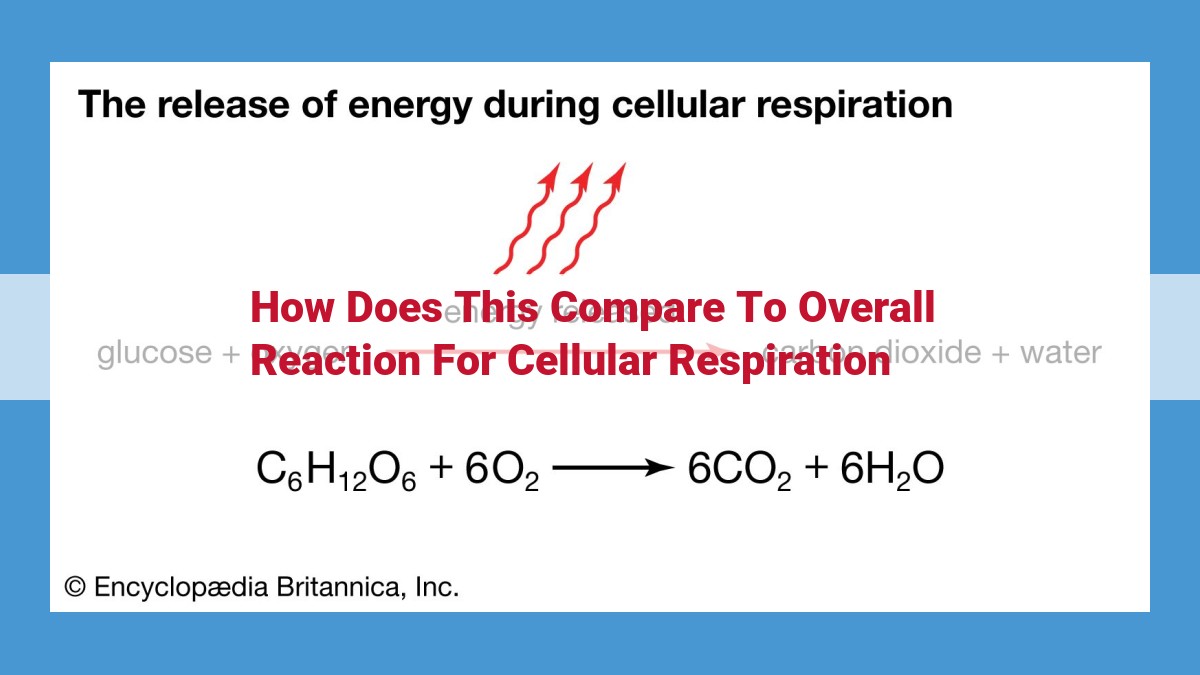
In the realm of cellular energy production, two distinct metabolic pathways reign supreme: anaerobic respiration and aerobic respiration. Both journeys begin with an unassuming molecule called glucose, the primary fuel source for these energy-yielding processes.
Glycolysis, the initial stage of both pathways, orchestrates the breakdown of glucose into smaller molecules. While anaerobic respiration relies solely on glycolysis for energy production, aerobic respiration uses it as a mere appetizer to kickstart a more elaborate energy feast.
Outputs: Fermentation and Byproduct Production
In the absence of oxygen, anaerobic respiration takes a different turn, leading to the intriguing process of fermentation. During this chemical transformation, glucose undergoes a series of reactions, culminating in the production of distinct fermentation products. These products are the key players behind the tantalizing flavors and aromas that characterize various fermented foods and beverages.
Let’s venture into the world of fermentation, where two prominent products emerge: ethanol and lactic acid. Ethanol, also known as alcohol, is the substance that gives alcoholic beverages their intoxicating effects and unique aromas. It’s the result of glucose’s conversion into pyruvate, followed by a reduction reaction that generates ethanol.
On the other hand, lactic acid is a tangy substance that contributes to the distinctive flavors of fermented dairy products like yogurt and cheese. It’s formed when pyruvate undergoes a different reduction reaction.
The presence of these fermentation products isn’t just a matter of taste; they also have physiological effects. Lactic acid fermentation, for instance, can lower the pH of the environment, creating an acidic barrier that inhibits the growth of harmful bacteria. This acidic environment enhances the preservation of fermented foods and beverages.
So, next time you savor the flavors of a fermented delicacy, remember the fascinating journey of glucose metabolism that brought it to life. The presence of ethanol or lactic acid is more than just a testament to the culinary artistry; it’s a testament to the intricate processes that transform simple sugars into the delights that tantalize our taste buds.
Energy Yield: Substrate-Level Phosphorylation vs. Electron Transport Chain
In the realm of cellular energy production, two pivotal pathways reign supreme: anaerobic respiration and aerobic respiration. While both utilize glucose as their primary fuel, a chasm exists between their outputs and efficiency.
Anaerobic Respiration: A Modest Energy Yield
In anaerobic respiration, the breakdown of glucose yields a mere 2 ATP molecules. This paucity stems from a limited energy-yielding mechanism known as substrate-level phosphorylation. Here, ATP is directly synthesized by transferring a high-energy phosphate group from an intermediate molecule to ADP.
Aerobic Respiration: An Energy Powerhouse
In stark contrast, aerobic respiration harnesses a vastly more potent energy-yielding mechanism: the electron transport chain. This intricate system generates a whopping 36-38 ATP molecules per glucose molecule. The electron transport chain operates in tandem with oxidative phosphorylation, where the energy released by electron transfer is used to pump protons across a membrane, creating an electrochemical gradient that drives the synthesis of ATP.
Why Aerobic Respiration Reigns Supreme
The stark difference in energy yield between anaerobic and aerobic respiration is a testament to the exceptional efficiency of the electron transport chain. Oxidative phosphorylation’s ability to extract far more energy from glucose through a series of coupled redox reactions sets aerobic respiration far ahead as the superior energy-producing pathway.
Location: Cytoplasm and Cellular Compartments
The journey of glucose, our body’s primary fuel source, begins in the cytoplasm, the bustling center of cellular activity. Here, anaerobic respiration takes the stage, a process that unfolds exclusively within this cellular realm. Through a series of enzymatic reactions, glucose is broken down into pyruvate, the key intermediate in energy metabolism.
In eukaryotic cells, a more complex dance unfolds. As oxygen becomes available, the drama shifts to the mitochondria, the powerhouses of the cell. Within these intricate compartments, aerobic respiration takes center stage. Glucose’s odyssey continues through a labyrinth of reactions, culminating in the electron transport chain, a marvel of efficiency that generates the bulk of our energy currency, ATP.
So, the cytoplasm and mitochondria serve as the primary battlegrounds for energy production in our cells. Anaerobic respiration, a more primitive process, reigns supreme in the cytoplasm. Aerobic respiration, far more efficient, ascends to the stage within the mitochondria, propelling us through our daily lives with its abundant energy supply.
Aerobic Respiration’s Superior Energy Efficiency
In the realm of energy production, aerobic respiration reigns supreme as the most efficient cellular process. Unlike its anaerobic counterpart, which yields a meager 2 ATP molecules per glucose molecule, aerobic respiration harnesses the power of oxygen to generate a whopping 36-38 ATP molecules, a staggering 20x increase in energy yield.
The secret to aerobic respiration’s exceptional efficiency lies in its intricate enzymatic machinery, particularly the electron transport chain and oxidative phosphorylation. These processes work in tandem to maximize ATP production through a series of redox reactions.
The electron transport chain, nestled within the folds of the mitochondrial membrane, acts as an energy conveyor belt. As electrons pass down this chain, their energy is captured and used to pump protons (H+) across the membrane, creating an electrochemical gradient. This gradient, like a miniature dam, drives protons back through a specialized protein called ATP synthase. As the protons flow through ATP synthase, their energy is harnessed to synthesize ATP from ADP and inorganic phosphate.
In essence, aerobic respiration exploits the energy released by electron transfer to generate ATP. The more efficient the electron transport chain and the higher the proton gradient, the more ATP can be produced. This explains why aerobic respiration, with its extensive electron transfer network and tightly coupled oxidative phosphorylation, far outperforms anaerobic respiration in terms of energy yield.
So, next time you’re feeling a burst of energy, remember the incredible efficiency of aerobic respiration powering your every move. This process is a testament to the ingenuity of life’s chemistry, enabling us to thrive in a world that demands constant energy.