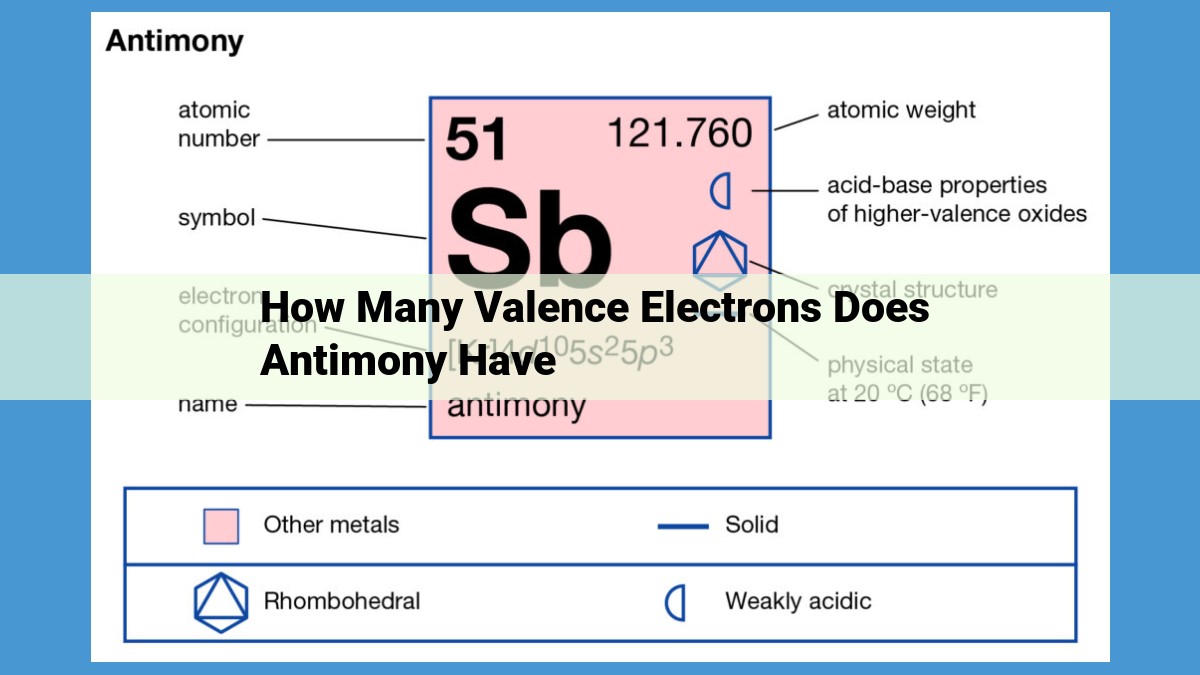
Antimony, a metalloid in Group 15, possesses five valence electrons. Valence electrons are the outermost electrons in an atom’s orbitals that participate in chemical bonding. Antimony’s electron configuration, [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³, reveals that it has three valence electrons in the 6p subshell and two in the 6s subshell, totaling five valence electrons. These electrons play a crucial role in antimony’s chemical reactivity, enabling it to form diverse compounds with other elements.
Antimony: Unraveling the Secrets of Valence Electrons
In the realm of chemistry, antimony stands as an enigmatic figure, a metalloid that resides in Group 15 of the periodic table. Its intricate electron configuration holds the key to understanding its unique properties and endless possibilities in the world of chemical reactions.
Our mission today is like embarking on a thrilling expedition, where we’ll unravel the secrets of antimony’s valence electrons. Brace yourself for a journey through the fascinating concepts of valence electrons, electron configuration, and the intricate world of quantum mechanics.
What Are Valence Electrons?
Valence electrons, those lone ranger electrons, hold the power to dictate the chemical behavior of an element. They reside in the outermost shell of an atom, yearning for companionship and eagerly awaiting the opportunity to participate in the dance of chemical bonding. These electrons play a critical role in defining an element’s reactivity and shaping its destiny in the world of chemistry.
Electron Configuration: The Blueprint of an Atom
Every atom, including our enigmatic antimony, possesses a unique electron configuration, a blueprint that reveals the arrangement of its electrons within various energy levels and orbitals. Picture this configuration as a celestial symphony, with electrons swirling around the nucleus like planets orbiting a star.
Antimony’s Electron Configuration: A Tale of Five
Antimony’s electron configuration, with its symphony of electrons, unfolds as follows: [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³. This enigmatic arrangement unveils a tale of five valence electrons, eager to participate in the grand scheme of chemical reactions.
The Significance of Antimony’s Valence Electrons
These five valence electrons are the driving force behind antimony’s remarkable versatility. They orchestrate the formation of chemical bonds, allowing antimony to form a myriad of compounds with diverse properties. From alloys that enhance the strength of steel to semiconductors that power our technological marvels, antimony’s valence electrons are the unsung heroes behind countless innovations.
In this captivating exploration, we’ve uncovered the secrets of antimony’s valence electrons. Understanding these electrons not only deepens our knowledge of this enigmatic metalloid but also provides a glimpse into the fundamental principles that govern the behavior of all elements. So, as you delve deeper into the world of chemistry, carry with you the knowledge of antimony’s valence electrons and the profound impact they have on the symphony of chemical reactions.
Valence Electrons: The Key Players in Chemical Bonding
Let’s unravel the fascinating world of valence electrons, the building blocks of chemical bonding. These mischievous electrons reside in the outermost energy level of an atom, eager to mingle and form bonds with their neighbors.
Their primary role is to determine how an element behaves chemically. By understanding the number and arrangement of valence electrons, scientists can predict an element’s bonding preferences and reactivity.
Imagine valence electrons as sprites, dancing around the nucleus like ethereal fairies. They occupy specific energy levels, called atomic orbitals. Each orbital represents a region of space where the electron is most likely to be found.
S orbitals are spherical, like soap bubbles, while p orbitals have distinct lobes, resembling dumbbells. The number and type of atomic orbitals in an energy level determine the number of valence electrons an element can have.
So, there you have it – an introduction to the enigmatic world of valence electrons. In the next chapter, we’ll explore the electron configuration of antimony, a metalloid with a unique and intriguing valence electron count.
Concept 2: Electron Configuration: The Blueprint of an Atom’s Electrons
In a journey to understand the captivating world of antimony, we delve into the concept of electron configuration. This intricate blueprint reveals the arrangement of electrons within an atom, offering crucial insights into its chemical behavior.
Electron configuration is a symbolic representation that depicts the distribution of electrons in an atom’s energy levels, or orbitals. Each orbital can hold a specific number of electrons, and the outermost electrons, called valence electrons, play a pivotal role in chemical bonding.
Two fundamental principles guide electron configuration:
- Aufbau Principle: Electrons fill the available orbitals in order of increasing energy.
- Hund’s Rule: Within an orbital, electrons occupy individual orbitals before pairing up.
Using Electron Configuration to Unravel Valence Electrons:
An atom’s electron configuration holds the key to uncovering its valence electrons. The valence electrons reside in the outermost energy level, and their number determines an atom’s chemical reactivity. To extract this information, we follow these steps:
- Start with the atomic number, which indicates the total number of electrons.
- Distribute the electrons in the orbitals based on the Aufbau principle and Hund’s rule.
- Identify the outermost energy level, which contains the valence electrons.
Example: Antimony’s Electron Configuration and Valence Electrons:
Antimony, an enigmatic element from Group 15, possesses an electron configuration of [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³. The inert xenon core, represented by [Xe], holds 54 electrons, leaving 10 electrons to be distributed in the outermost energy levels.
Applying the Aufbau principle, we fill the 5d orbitals with 10 electrons and the 6s orbitals with 2 electrons. The remaining 3 electrons enter the 6p orbitals, which are the valence orbitals. Therefore, antimony has five valence electrons.
Understanding the concept of electron configuration and its profound impact on valence electrons empowers us with the knowledge to unravel the chemical mysteries of antimony and countless other elements.
Concept 3: Antimony’s Electron Configuration
Understanding Valence Electrons
Before delving into Antimony’s specific electron configuration, let’s recap the concept of valence electrons. These are the outermost electrons in an atom, responsible for chemical bonding and determining an element’s reactivity. Picture them as the social butterflies of the atomic world, always eager to interact with other atoms.
Electron Configuration: A Map to Valence Electrons
Electron configuration is a roadmap depicting the distribution of electrons in an atom’s orbitals. It follows two guiding principles:
- Aufbau Principle: Electrons fill orbitals in increasing energy levels.
- Hund’s Rule: Orbitals of equal energy are filled with unpaired electrons before pairing occurs.
Antimony’s Electron Configuration: Unveiling the Valence
Now, let’s unravel Antimony’s specific electron configuration: [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³. This notation represents a shorthand for the distribution of electrons in its orbitals.
[Xe]: This symbolizes Antimony’s core electrons, which are the same as Xenon’s (atomic number 54).4f¹⁴ 5d¹⁰: These represent the filled inner orbitals.6s² 6p³: Here’s the key! These are Antimony’s valence electrons, two in the 6s orbital and three in the 6p orbital.
The Magic Number: Five Valence Electrons
Antimony’s five valence electrons play a pivotal role in its chemical behavior. They determine the number of bonds it can form and the types of compounds it can create. These electrons are the gateway to understanding Antimony’s reactivity and its interactions with other elements.
Answering the Question:
- Valence Electrons Count: Five
Delving into the Enigmatic Valence Electrons of Antimony: Unveiling the Chemical Bonding Secrets
In the realm of elements, we encounter the intriguing world of antimony, a metalloid that resides in Group 15 of the periodic table. To fully grasp its chemical bonding prowess, we embark on a journey to determine antimony’s valence electrons, the key players in forming chemical bonds and defining its reactivity.
Valence Electrons: The Gateway to Chemical Bonding
Valence electrons, the electrons residing in the outermost energy level of an atom, dictate an element’s chemical behavior. They are the gatekeepers of chemical bonding, playing a crucial role in determining how an element interacts with others. To uncover antimony’s valence electrons, we must delve into the fascinating world of electron configuration.
Electron Configuration: Unraveling the Subatomic Architecture
Electron configuration refers to the distribution of electrons across different energy levels or orbitals. This arrangement is guided by two fundamental principles: the Aufbau Principle, which dictates the sequential filling of orbitals, and Hund’s Rule, which favors the spread of electrons across orbitals.
Antimony’s Electron Configuration: A Tale of Five Valence Electrons
Antimony’s electron configuration, represented by [Xe] 4f¹⁴ 5d¹⁰ 6s² 6p³, unveils its unique atomic structure. The configuration reveals that antimony possesses five valence electrons, generously bestowed upon it in the outermost 6s² and 6p³ orbitals.
The Significance of Antimony’s Valence Electrons
Antimony’s five valence electrons play a pivotal role in its chemical reactivity. These electrons eagerly participate in bonding, forming covalent bonds with other elements to achieve a stable and balanced state. The presence of these valence electrons enables antimony to exhibit a wide range of chemical interactions, making it a versatile element in various applications.
Our exploration into the valence electrons of antimony has unveiled the fundamental principles that govern its chemical bonding behavior. With five valence electrons at its disposal, antimony exhibits a multifaceted reactivity, contributing to its diverse uses in the fields of electronics, alloys, and pharmaceuticals. The understanding of valence electrons empowers us to predict and manipulate the chemical properties of elements, unlocking the secrets of the atomic world.