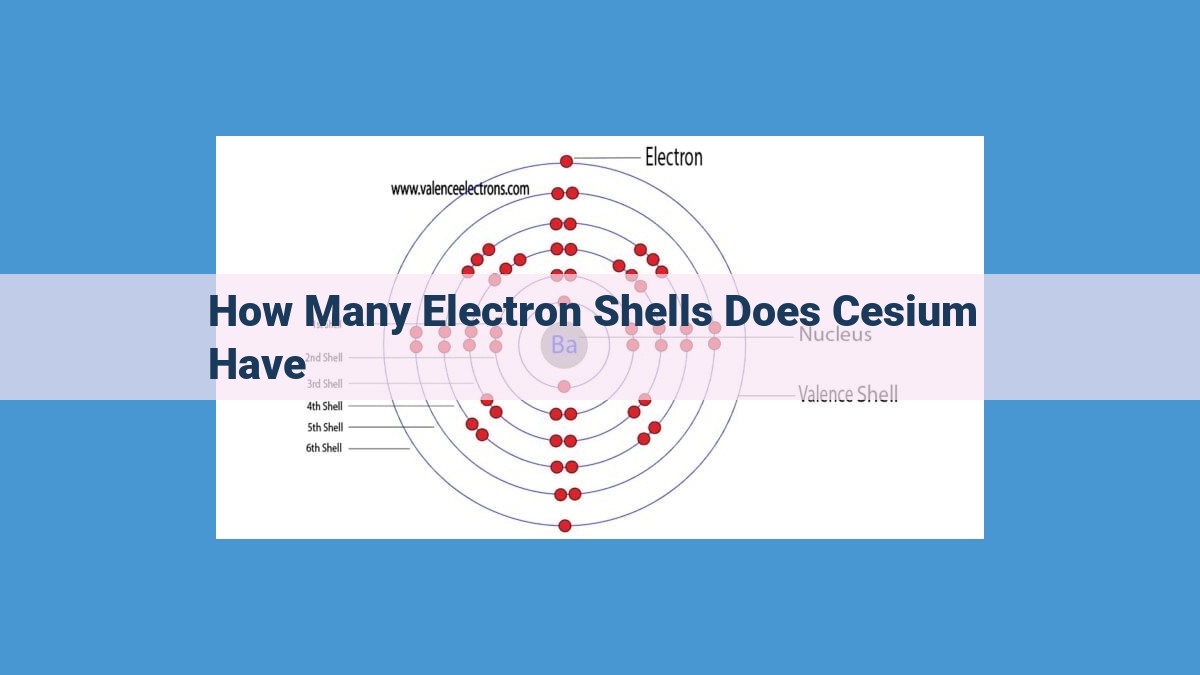
- Cesium, with an atomic number of 55, has six electron shells.
Electron Shell Essentials: Laying the Foundation for Chemistry’s Dance
The atom, the building block of all matter, holds a captivating secret within its core: electron shells. These invisible layers, swirling like electron clouds, dictate the behavior and properties of elements that shape our world. Understanding electron shells is a pivotal step in unlocking the mysteries of chemistry.
Electron Shells and Their Impact
Electron shells are discrete regions around the atom’s nucleus where electrons reside. Valence electrons, those occupying the outermost shell, play a crucial role in chemical bonding. Their behavior determines the atom’s reactivity and ability to form bonds with other atoms.
By understanding the number and arrangement of valence electrons, scientists can predict the chemical properties of elements and explain their behavior in reactions. These versatile electrons are responsible for the diverse array of molecules and compounds that make up our universe.
Atomic Number: The Nucleus’s Heart
In the realm of atoms, the atomic number reigns supreme as the fundamental identifier that sets each element apart. It’s like a cosmic fingerprint, uniquely defining the very essence of an element. The atomic number, denoted by the symbol Z, reveals the number of positively charged protons residing within the nucleus, the atom’s central core.
But the atomic number doesn’t work alone. It shares a close kinship with the proton number, which, unsurprisingly, also equals Z. This harmonious relationship stems from the fact that protons are the only subatomic particles carrying a positive electrical charge, and the atomic number represents the net positive charge of the nucleus.
Alongside protons, the nucleus also houses electrically neutral neutrons, which contribute to the atom’s mass. The combined number of protons and neutrons, denoted as mass number, provides a measure of the atom’s overall mass.
The atomic number plays a pivotal role in organizing the elements in the iconic Periodic Table. Elements are arranged in ascending order of their atomic numbers, creating a systematic chart that showcases their properties and relationships. This ordering is no mere coincidence; it reflects the fundamental structure of each element’s atom, with the number of protons dictating the number of electrons that orbit the nucleus.
In the broader scheme of chemistry, the atomic number serves as the compass that guides our understanding of an element’s behavior. It governs the element’s chemical reactivity, determining whether it will readily form bonds with other elements. The atomic number also influences an element’s ionization energy, the amount of energy required to remove an electron, and its electronegativity, a measure of its ability to attract electrons.
Understanding the atomic number is akin to deciphering the genetic code of an element. It unlocks the secrets of its identity, its properties, and its place within the vast tapestry of the Periodic Table.
Noble Gas Nirvana: Electron Stability Unraveled
In the realm of chemistry, stability is a holy grail, and noble gases stand as the epitome of electronic tranquility. These enigmatic elements, tucked away at the far right of the periodic table, possess a secret that unlocks the key to chemical harmony—a stable electron configuration.
Imagine electrons as tiny dancers, twirling in a quantum waltz around the atomic nucleus. Noble gases, like elegant ballerinas, have achieved a perfect balance, with their electron shells completely filled. This harmonious arrangement grants them an unwavering stability, making them reluctant to participate in the dance of chemical reactions.
The importance of achieving a noble gas electron configuration in chemical reactions cannot be overstated. It’s like a cosmic dance floor, where atoms strive to find their perfect match. When atoms interact, they exchange or share electrons in a bid to mimic the stable electron arrangement of noble gases. This noble gas Nirvana drives the formation of chemical bonds, creating the vast tapestry of molecules that make up our world.
So, the next time you witness a chemical reaction, remember the silent symphony of electrons behind the scenes, seeking their own noble gas Nirvana. It’s a testament to the fundamental principles that govern the dance of atoms and the stability that underpins the very fabric of our universe.
Electron Configuration: A Quantum Dance
Embark on a journey into the microscopic realm where electrons perform an intricate dance within atoms. Dive into the symphony of quantum numbers and orbitals, unveiling the secrets of electron configuration.
Orbitals, the Electron’s Abode
Electrons, nature’s mischievous dancers, reside in designated abodes called orbitals. Visualize them as three-dimensional regions around the atomic nucleus, each with its unique shape and energy level. Consider the s orbital, a spherical realm where the electron encircles the nucleus like a shy shadow. Contrast this with the dumbbell-shaped p orbitals, their lobes stretching out in three different directions.
Quantum Numbers: Describing the Dance
Each electron’s dance is governed by a quartet of quantum numbers:
- Principal quantum number (n): Defines the energy level, with higher numbers indicating greater distance from the nucleus.
- Azimuthal quantum number (ℓ): Determines the orbital shape, denoting s, p, d, or f orbitals.
- Magnetic quantum number (mℓ): Specifies the orientation of the orbital in space, affecting its magnetic properties.
- Spin quantum number (ms): Describes the electron’s inherent spin, either “up” or “down.”
The Symphony of Electron Configuration
Electron configuration is the blueprint of the electron dance, revealing which orbitals are occupied and how many electrons reside in each. It dictates the atomic properties that shape the behavior of elements, such as reactivity and ionization energy.
Delving into the Details
- s orbitals: Accommodate a maximum of 2 electrons, forming the foundation of atoms.
- p orbitals: Hold up to 6 electrons, shaping the outermost electron shell and influencing chemical bonding.
- d orbitals: Can accommodate 10 electrons, playing a crucial role in transition metals and their unique properties.
- f orbitals: The largest orbitals, capable of holding 14 electrons, found in elements deep within the periodic table.
By understanding electron configuration, we unravel the quantum ballet performed by electrons, unlocking the secrets that govern the behavior of matter at the atomic level.
Periodic Trends: A Tale of Elements’ Variations
- Describe periodic trends in ionization energy, electron affinity, electronegativity, and atomic radius.
- Explain how these trends relate to the properties of elements and their positions in the periodic table.
Periodic Trends: Unraveling the Symphony of Elements
In the vast cosmic tapestry, elements dance in an intricate ballet, each endowed with unique traits that mold their behavior and determine their place in the periodic table. Among these traits, ionization energy, electron affinity, electronegativity, and atomic radius stand out as orchestrators of this elemental symphony.
Ionization Energy: The energy required to remove an electron from an atom’s outermost valence shell reveals its resistance to losing electrons. Elements on the far right of the periodic table, like Helium, possess high ionization energies due to their tightly bound electrons, while those on the far left, such as Cesium, have low ionization energies, readily parting with their electrons.
Electron Affinity: The change in energy when an atom gains an electron into its outermost shell showcases its eagerness to accommodate more electrons. Elements on the top right corner, such as Fluorine, exhibit high electron affinities, eagerly welcoming electrons into their empty orbitals. In contrast, elements on the bottom left corner, like Radon, have low electron affinities, displaying little enthusiasm for gaining electrons.
Electronegativity: This property quantifies an atom’s “grasping power” for electrons in a chemical bond. Elements high up and to the right in the periodic table, like Oxygen, are highly electronegative, fiercely pulling electrons toward themselves. On the other hand, elements low down and to the left, like Sodium, are less electronegative, willing to share their electrons more generously.
Atomic Radius: The distance from an atom’s nucleus to its outermost electron shell influences its overall size. Elements on the left and bottom of the periodic table tend to have larger atomic radii due to more electron shells, while those on the right and top have smaller atomic radii due to fewer electron shells.
These periodic trends are not mere abstract concepts; they play a pivotal role in shaping the properties of elements and dictating their reactivity in chemical reactions. They guide the formation of molecules, the flow of electricity, and the very existence of life on Earth. By understanding these trends, we unlock the secrets of the periodic table, revealing the underlying patterns that govern the behavior of all matter.
Cesium: A Study in Reactivity
In the realm of elements, cesium stands out as a captivating subject due to its exceptional reactivity. This intriguing element holds a unique place in the periodic table, and its remarkable properties offer valuable insights into the fundamentals of chemistry.
Atomic Profile of Cesium
Cesium, with the symbol Cs, possesses an atomic number of 55. This number signifies the presence of 55 protons and electrons within its atomic structure. Its electron configuration, which describes the arrangement of these electrons, is [Xe] 6s¹. This configuration indicates that cesium has one valence electron in its outermost electron shell.
Why Cesium is Highly Reactive?
The high reactivity of cesium can be attributed to two key factors:
-
Low Ionization Energy: Ionization energy refers to the amount of energy required to remove an electron from an atom. Cesium possesses a very low ionization energy, making it easy for its outermost electron to be removed. This low ionization energy allows cesium to readily undergo chemical reactions.
-
Low Electronegativity: Electronegativity measures an atom’s ability to attract electrons. Cesium has a low electronegativity, indicating its weak ability to attract electrons. This low electronegativity makes it more likely for cesium to lose its valence electron, contributing to its high reactivity.
Applications of Cesium’s Reactivity
The exceptional reactivity of cesium has led to its utilization in various applications:
-
Atomic Clocks: Cesium’s stable and predictable atomic transitions are used in highly accurate atomic clocks, which are vital for precise timekeeping and navigation systems.
-
Ion Thrusters: Cesium ions are employed as propellant in ion thrusters, a type of spacecraft propulsion system that provides efficient and low-thrust propulsion.
-
Photocells: Cesium is used as a photosensitive material in photocells, which convert light into electrical energy.
Cesium’s high reactivity, stemming from its low ionization energy and electronegativity, has made it an element of significant interest in scientific research and technological advancements. By understanding the intricacies of its reactivity, we gain valuable insights into the fundamental principles that govern the behavior of matter.