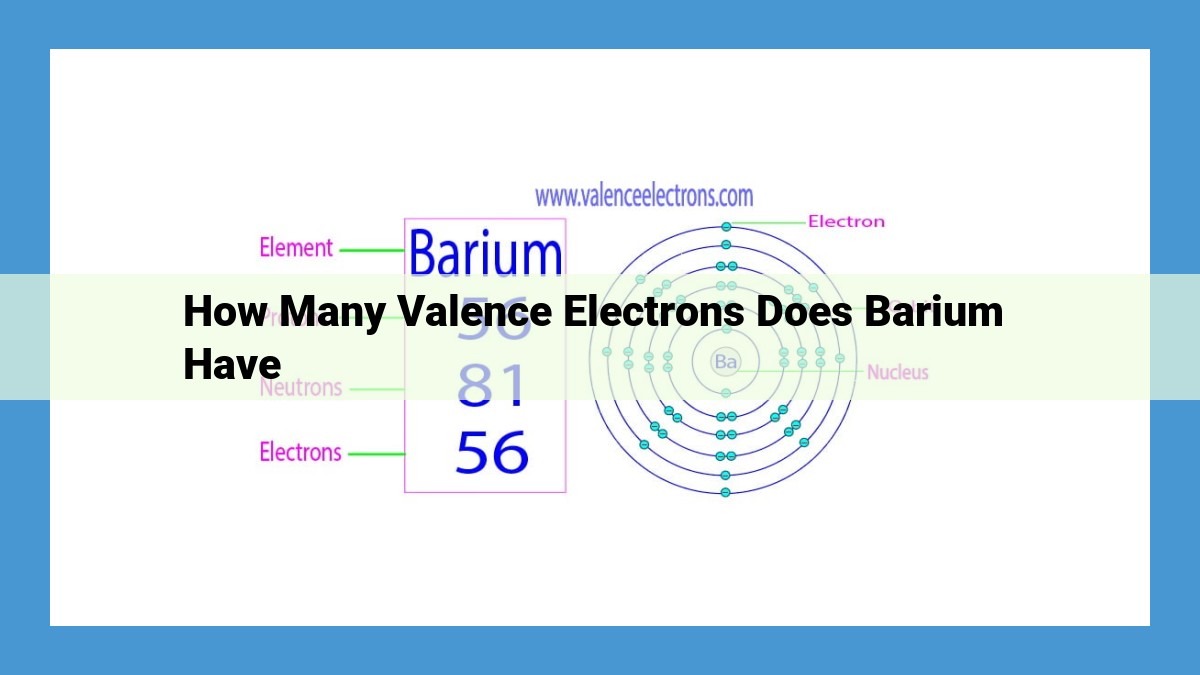
Barium, an alkaline earth metal located in Group 2 of the periodic table, possesses two valence electrons. Its atomic number of 56 indicates that it has 56 electrons, with the two valence electrons residing in the outermost 6s orbital. These valence electrons play a crucial role in determining barium’s chemical properties, enabling it to form various compounds, such as barium oxide, barium chloride, and barium sulfate, due to its tendency to lose these electrons and achieve a stable octet configuration.
Understanding Valence Electrons
Have you ever wondered why different elements combine in specific ways to form the amazing world around us? To understand this fascinating chemistry, let’s delve into the concept of valence electrons, the key players that determine the chemical behavior of an element.
Valence electrons are the electrons located in an atom’s outermost energy level. They are the most reactive electrons and the ones involved in forming chemical bonds with other atoms. Think of them as the atom’s hands, reaching out to interact with their neighbors.
The number of valence electrons an element has significantly influences its chemical properties. Metals like sodium or potassium have one or two valence electrons, making them eager to donate or shed these electrons, forming positive ions. Nonmetals, like chlorine or oxygen, have five to seven valence electrons, seeking to gain electrons and form negative ions. These electron exchanges drive the formation of the countless compounds and molecules that make up our world.
Barium’s Position in the Periodic Table
- Discuss barium’s location in Group 2 of the periodic table and its relationship to other alkaline earth metals.
Barium’s Position in the Periodic Table
Prepare yourself for a fascinating journey through the world of chemistry as we delve into the captivating tale of barium’s unique position in the periodic table.
Imagine a magnificent tapestry woven with intricate threads of elements, each with its own distinct personality. Among this vibrant ensemble, barium, an alkaline earth metal, stands out like a shining star.
Group 2: The Family of Alkaline Earth Metals
Barium proudly resides in Group 2 of the periodic table, keeping company with other alkaline earth metals, such as magnesium, calcium, and strontium. These elements share a common trait: each possesses two valence electrons, the very electrons that determine their chemical characteristics.
The Tale of Group 2
Like close-knit family members, the alkaline earth brothers share certain similarities. They exhibit a strong affinity for losing their two valence electrons to form stable positive ions. This two-electron donation plays a pivotal role in shaping their chemistry.
Barium’s Identity: A Balancing Act
Now, let’s focus on our protagonist, barium. With an atomic number of 56, it boasts 56 electrons diligently orbiting its nucleus. Thirty-two of these electrons form four energy levels, while the remaining two reside in the fifth and outermost energy level, known as the valence shell. These two valence electrons give barium its distinctive chemical identity.
Connections to Its Neighbors
Barium stands shoulder-to-shoulder with its Group 2 brethren. It resembles calcium in many ways, but it also showcases subtle differences. For instance, barium exhibits a greater reactivity due to its larger atomic size and lower ionization energy.
Summary
In conclusion, barium’s position in Group 2 of the periodic table profoundly influences its chemical nature. With its two valence electrons, barium eagerly reacts to form stable ions, a characteristic shared by its alkaline earth metal family. By understanding barium’s unique place in the periodic table, we gain insights into its intriguing world of chemistry.
Atomic Number and Electron Configuration of Barium
In the realm of chemistry, unraveling the secrets of elements is a captivating pursuit. Let’s embark on a journey to understand Barium, an element nestled in Group 2 of the periodic table. Like a passport that unveils a traveler’s identity, the atomic number serves as a unique identifier for each element, dictating the number of protons and electrons it possesses.
For Barium, this atomic number is 56, a number that holds the key to its chemical personality. The arrangement of electrons around an atomic nucleus, known as its electron configuration, further defines its characteristics. Barium’s electron configuration is a captivating tale, encapsulated within the notation [Xe]6s².
Picture this: deep within Barium’s atomic structure, electrons whirl around the heart of the atom, the nucleus. The [Xe] portion of its electron configuration represents a stable core of electrons, resembling the noble gas Xenon. Beyond this core, two solitary electrons occupy the outermost shell, dancing in the 6s orbital.
These valence electrons play a pivotal role in determining Barium’s chemical destiny. They are the messengers that connect Barium to other elements, allowing it to form bonds and participate in the intricate dance of chemical reactions. As we delve deeper into Barium’s world, we will witness the remarkable influence of these valence electrons, shaping its behavior and revealing its chemical prowess.
Valence Electrons of Barium: Understanding the Chemical Magic
In the enchanting tapestry of the periodic table, barium holds a prominent place as an alkaline earth metal in Group 2. Its unique position and atomic number of 56 endow it with specific characteristics that set it apart from its elemental brethren.
Valence Electrons: The Key to Chemical Behavior
Every atom, like a miniature solar system, consists of a nucleus surrounded by electrons. The valence electrons are those occupying the outermost energy level, and they play a pivotal role in determining an element’s chemical behavior.
Barium possesses two valence electrons, residing in the 6s orbital. These outermost electrons are like eager participants in the dance of chemical reactions, ready to engage with other atoms to form bonds and create new substances.
Significance of Valence Electrons for Barium
The presence of these two valence electrons grants barium remarkable properties:
- Chemical Reactivity: The valence electrons make barium highly reactive, readily forming bonds with other elements.
- Metallic Nature: The mobile valence electrons allow barium to conduct electricity and heat, characteristic of metals.
- Ionic Bonding: Barium readily loses its valence electrons, forming positive ions with a charge of +2.
Barium Compounds: A Testament to Valence Electrons
Barium’s valence electrons enable it to form an array of compounds with diverse applications:
- Barium Oxide (BaO): Used in the production of refractory materials and ceramics.
- Barium Chloride (BaCl₂): Employed as a flux in smelting operations and as a purifying agent in water treatment.
- Barium Sulfate (BaSO₄): Commonly known as “barytes,” it serves as a drilling fluid additive and a contrast agent in medical imaging.
In conclusion, barium’s valence electrons are the driving force behind its chemical prowess. They determine its reactivity, metallic nature, and ability to form various compounds. Understanding these valence electrons enables us to appreciate the intricacies and wonders of the elemental world.
Related Concepts: Delving into the World of Valence Electrons
To truly grasp the significance of barium’s valence electrons, it’s essential to delve into related concepts that paint a broader picture of their role in shaping the element’s chemical behavior.
Atomic Orbitals: The Electron’s Abode
Imagine electrons as tiny dancers swirling around the nucleus of an atom. These dancers reside in specific atomic orbitals, which are regions where they’re most likely to be found. Barium’s valence electrons occupy the 6s orbital, resembling a cloud of energy surrounding the nucleus.
Covalent Bonds: The Glue that Binds
Valence electrons play a crucial role in forming covalent bonds—the chemical bonds that unite atoms. When two atoms share their valence electrons, they create a strong bond that holds them together. In barium’s case, its two valence electrons enable it to form stable covalent bonds with other atoms.
Chemical Reactivity: The Dance of Electrons
The number and arrangement of valence electrons determine an element’s chemical reactivity. Elements with more valence electrons tend to be more reactive, while those with fewer valence electrons are less reactive. Barium, with its two valence electrons, exhibits moderate reactivity, allowing it to participate in various chemical reactions.
Alkaline Earth Metals: A Family of Similarities
Barium belongs to the alkaline earth metals, a group of elements located in Group 2 of the periodic table. These metals share similar properties, including two valence electrons and a tendency to form ionic bonds by losing these electrons.
Group 2 Elements: A Shared Destiny
In the periodic table, elements are organized into groups based on their number of valence electrons. Barium is part of Group 2, also known as the alkaline earth metal group. This group consists of elements that all have two valence electrons, influencing their chemical behavior and the compounds they form.
Barium Compounds: The Significance of Valence Electrons
In our exploration of barium’s fascinating world, we’ve delved into its valence electrons. Now, let’s unravel the captivating story of how these electrons enable barium to forge diverse compounds and shape our technological landscape.
Barium Oxide: Illuminating the Night Skies
Barium’s valence electrons find their playmate in oxygen, forming barium oxide. This white, crystalline solid possesses an intriguing property: when heated, it emits a brilliant green light. This characteristic has earned it a starring role in fireworks, illuminating night skies with its enchanting glow.
Barium Chloride: From Medicine to Metalworking
When barium mingles with chlorine, barium chloride is born. This colorless compound finds its niche in medical imaging, acting as a contrast agent to enhance X-ray visibility. It also plays a pivotal role in metalworking, contributing to the heat treatment of steel and the production of aluminum.
Barium Sulfate: A Versatile Workhorse
The combination of barium and sulfur yields barium sulfate, a versatile compound with numerous applications. It serves as an inert filler in pigments, lending them opacity and whiteness. In the medical realm, it’s renowned as a contrast agent for gastrointestinal examinations. Moreover, it finds use in oil drilling as a heavy fluid to balance pressure.
The story of barium compounds underscores the profound impact of valence electrons on chemical behavior. By understanding these electrons and their interactions, we gain insights into the properties and applications of these remarkable substances. From illuminating skies to enhancing medical imaging, barium compounds continue to captivate us with their versatility and technological significance.