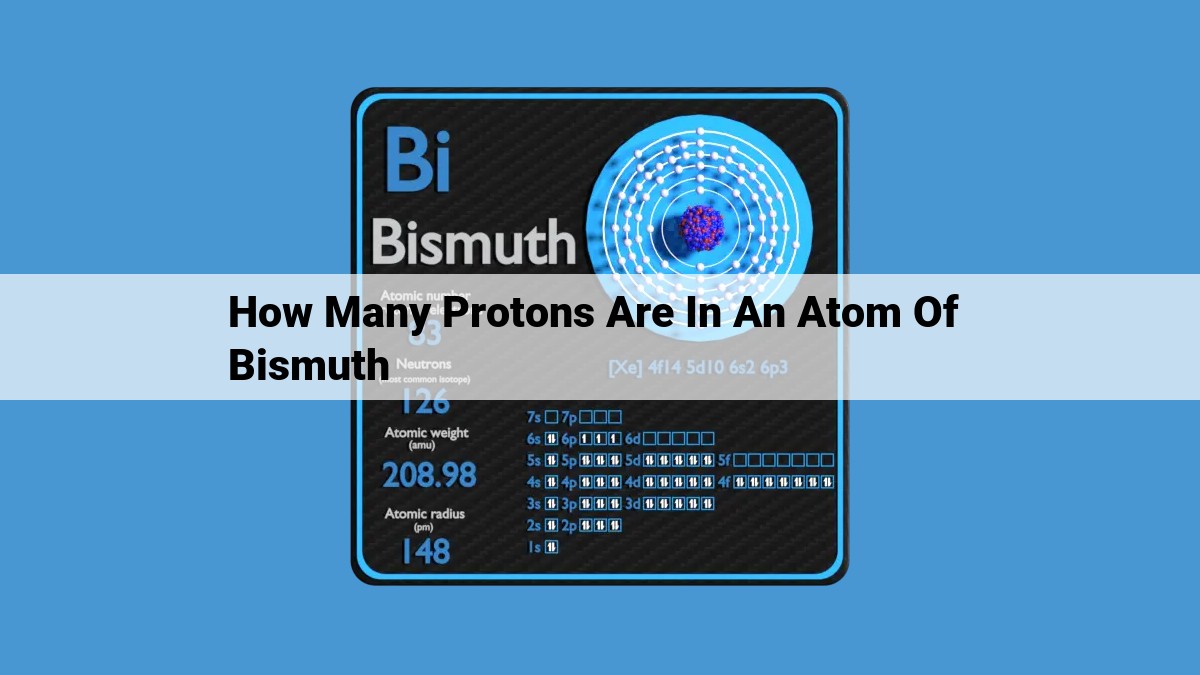
Bismuth, an intriguing element with the atomic number 83, boasts an atomic structure filled with 83 protons. This specific number of protons plays a pivotal role in shaping bismuth’s distinctive characteristics, including its chemical reactivity and nuclear stability. Understanding the atomic number of 83 in bismuth allows scientists to unravel the element’s behavior within various chemical reactions and comprehend its applications in diverse fields, such as pharmacology and materials science.
Understanding Atomic Number: The Key to Element Identity
In the vast tapestry of the universe, matter is composed of fundamental building blocks known as elements. Each element possesses a unique identity determined by its atomic number, a crucial concept that governs the very essence of its being.
Atomic Number: Nature’s Identifier
The atomic number of an element is the number of protons found within its atomic nucleus. This value not only distinguishes one element from another but also plays a central role in defining its chemical behavior. For instance, the element sodium (Na) has an atomic number of 11, signifying that its nucleus contains 11 protons. This distinct number sets sodium apart from all other elements, making it an essential component in various chemical reactions.
The Element Symbol: A Reflection of Atomic Number
The element symbol, a single or two-letter abbreviation, provides a concise representation of an element. This symbol bears a direct connection to the atomic number. For example, the element with an atomic number of 6 is carbon, symbolized as “C.” This simple notation allows scientists to effortlessly communicate and identify elements, facilitating seamless discussions on the composition of matter.
Balancing the Atom: Electrons and Nuclear Charge
The number of protons within an atom’s nucleus directly determines the number of electrons orbiting its nucleus. In a neutral atom, the positive charge of the protons is balanced by the negative charge of the electrons. This delicate equilibrium ensures the overall neutrality of the atom.
Furthermore, the nuclear charge, which represents the total positive charge of the protons in the nucleus, also correlates with the atomic number. This charge exerts a strong influence on the atom’s electron configuration and its subsequent chemical properties.
By comprehending the intricate relationship between atomic number, protons, electrons, and nuclear charge, we gain a deeper understanding of the fundamental nature of elements, enabling us to appreciate their unique contributions to the chemistry of our world.
Protons: The Fundamental Building Blocks of Atoms
In the vast universe of atoms, the smallest units of matter, protons play a crucial role in defining the very essence of an element. They are the fundamental building blocks that determine the element’s identity, its chemical properties, and its behavior in the world.
The atomic number of an element, denoted by Z, represents the number of protons in its nucleus. This value is unique to each element and serves as a key identifier. It is the atomic number that distinguishes, say, hydrogen (with one proton) from helium (with two protons).
The number of protons also has a direct impact on the number of electrons an atom possesses. Protons, with their positive charge, attract electrons, which carry a negative charge, to the atom’s electron cloud. The number of protons and electrons in an atom are equal in a stable state, maintaining electrical neutrality.
Furthermore, protons play a significant role in the nuclear energy of an atom. The nucleus, which resides at the center of the atom, contains protons and neutrons. The strong nuclear force, a powerful force that acts only over very short distances, binds these particles together. The number of protons influences the strength of this force and hence the stability of the atom.
Bismuth: A Case Study of 83 Protons
In the realm of elements, bismuth stands out as a captivating character with a unique atomic identity etched by its 83 protons. This enigmatic element holds a captivating story that unfolds as we delve into its atomic structure and explore the remarkable consequences of its protonic abundance.
Bismuth’s Atomic Identity: A Portrait of Distinction
Bismuth’s atomic number of 83 serves as a defining characteristic that sets it apart from all other elements. Embedded within the nucleus of each bismuth atom lie 83 protons, positively charged particles that dictate the element’s fundamental properties. This protonic endowment grants bismuth its unique position on the periodic table, nestled in the fifth period and fifteenth group.
The Dance of Protons: Shaping Bismuth’s Character
Protons play a pivotal role in shaping bismuth’s exceptional nature. The number of protons within an atom determines an element’s chemical behavior. Bismuth’s proton-rich nucleus exerts a strong electrostatic attraction on its orbiting electrons, resulting in a relatively high electronegativity. This enhanced electron-pulling ability renders bismuth less likely to give up its electrons, affecting its reactivity and chemical bonding tendencies.
Furthermore, the abundance of protons in bismuth’s nucleus influences its atomic weight and mass number. The combined mass of protons and neutrons in its nucleus gives bismuth a substantial atomic weight of 208.9804. This hefty atomic mass contributes to bismuth’s high density and solid state at room temperature.
From Proton Abundance to Chemical Consequences
Bismuth’s elevated proton count manifests itself in a myriad of chemical consequences. Its high electronegativity influences its bonding preferences, favoring the formation of covalent bonds with other electronegative elements such as chlorine and oxygen. Bismuth’s reluctance to part with its electrons makes it relatively less reactive, exhibiting lower chemical reactivity compared to other metals.
In addition, bismuth’s proton-packed nucleus endows it with a fascinating property known as diamagnetism. Unlike paramagnetic or ferromagnetic materials that are attracted to magnetic fields, bismuth exhibits the opposite behavior, repelling magnetic fields. This unique magnetic property stems from the paired electrons in bismuth’s atomic orbitals, which cancel each other’s magnetic moments.
As we unravel the tale of bismuth and its 83 protons, we discover an element of captivating complexity and allure. Its atomic identity, shaped by its protonic abundance, gives rise to a distinctive set of properties that make bismuth an intriguing subject for scientific exploration and technological applications.