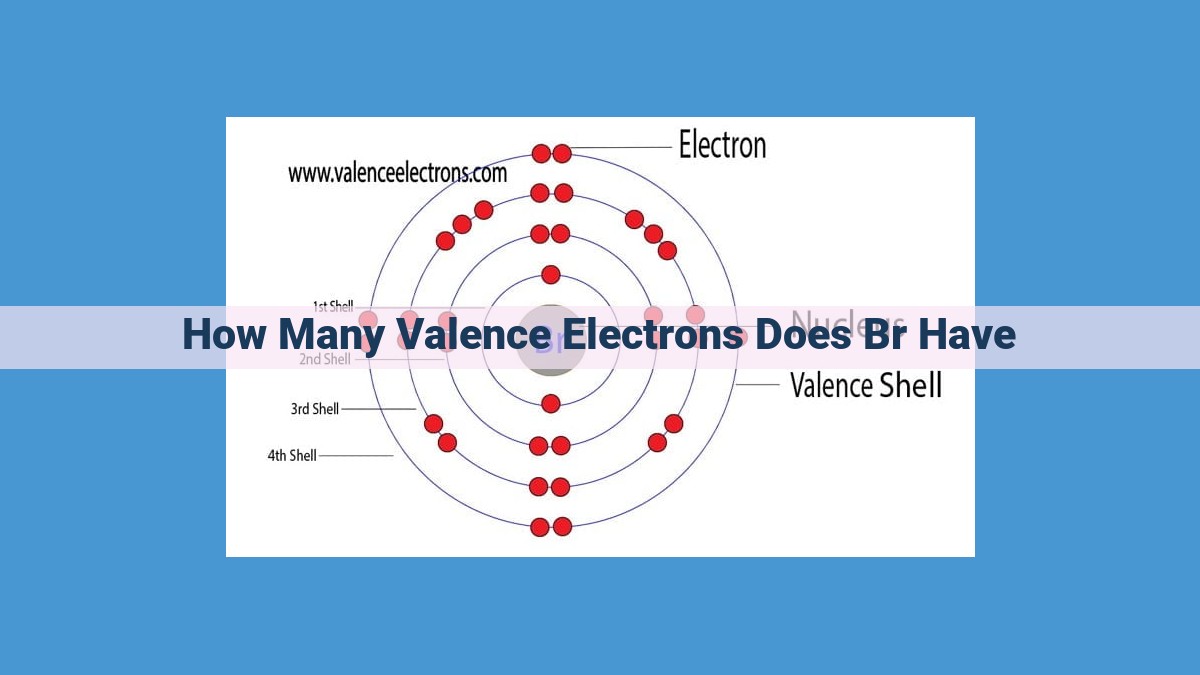
Bromine, a reactive halogen, has a significant number of valence electrons that play a crucial role in its chemical behavior. Located in the periodic table’s Group 17, bromine’s atomic number determines the total number of electrons, revealing the presence of seven valence electrons. These valence electrons occupy the outermost energy level, enabling bromine to readily form chemical bonds and participate in various reactions, shaping its reactivity and influencing its properties.
Understanding Valence Electrons: The Building Blocks of Chemical Reactions
At the very heart of chemistry, where elements dance in a cosmic waltz, lies the enigmatic concept of valence electrons. These are the electrons that reside in the outermost energy level of an atom, holding the key to its chemical behavior.
Think of valence electrons as the social butterflies of the atom, eager to mingle and interact with their neighbors. They are the ambassadors that form alliances, creating the bonds that hold molecules together. Their presence or absence determines whether an element is a shy introvert or a gregarious extrovert in the chemical world.
Valence electrons are found in atomic orbitals, which can be visualized as regions of space where they are most likely to be found. These orbitals are like tiny energy clouds surrounding the atomic nucleus, each with a unique shape and orientation. The closer an orbital is to the nucleus, the lower its energy.
As we explore the fascinating world of chemistry, understanding valence electrons is like holding the compass that guides us through the complexities of chemical reactions. These energetic particles are the driving force behind the formation, breakdown, and transformation of countless substances that shape our world.
Understanding Bromine: A Reactive Halogen with Unique Properties
In the realm of chemistry, the concept of valence electrons holds immense significance, influencing the reactivity and behavior of elements. Among the various elements, bromine stands out as a prime example, showcasing the crucial role of valence electrons in shaping its properties.
Bromine: A Reactive Halogen
Bromine, a member of the halogen group, is a highly reactive non-metallic element. Its intense reddish-brown color and pungent odor are characteristic of its strong oxidizing nature. Bromine exists primarily as a liquid at room temperature, and its reactivity stems from the presence of unpaired electrons in its outermost energy level. These unpaired electrons eagerly form bonds with other elements, making bromine a powerful oxidizing agent.
Classification in the Halogen Group
Halogens, a group of chemically similar elements located in Group 17 of the periodic table, exhibit a strong affinity for electrons. Bromine’s position in this group reflects its shared characteristics with other halogens, such as fluorine, chlorine, and iodine. These elements are highly reactive, forming salts with metals and displaying a range of oxidizing properties.
The Significance of Valence Electrons in Bromine
The number of valence electrons in an atom determines its chemical reactivity. For bromine, the number of valence electrons is crucial in understanding its behavior in chemical reactions. Valence electrons occupy the outermost energy level of an atom and participate in chemical bonding. By understanding the valence electron configuration of bromine, we can predict its bonding patterns and reactivity.
Bromine, an intriguing element within the halogen group, epitomizes the significance of valence electrons in chemistry. Its unique properties stem from the interplay between its valence electrons and its position in the periodic table. By exploring the fundamental concepts of valence electrons and their role in shaping the characteristics of bromine, we gain a deeper appreciation for the intricacies of the chemical world.
The Periodic Table: A Guide to Valence Electrons
Embarking on a Quest for Chemical Understanding
The periodic table, an iconic tool in chemistry, serves as a roadmap of the elements. It organizes them in a way that unveils their fascinating properties and behaviors. Among these elements lies bromine, a reactive nonmetal that holds a special place in this chemical tapestry.
Unraveling Bromine’s Atomic Secrets
To delve into the world of bromine, we must first uncover its position within the periodic table. It resides in Group 17, a group of elements aptly named the halogens. This group is known for its high reactivity, eager to form bonds with other elements. Bromine, like its halogen companions, possesses seven valence electrons—a crucial factor in determining its chemical behavior.
Valence Electrons: The Key to Reactivity
Valence electrons are the outermost electrons in an atom, and they play a pivotal role in chemical reactions. They are the explorers, venturing beyond the atom’s core to form bonds with other atoms. The number of valence electrons determines an element’s group number in the periodic table.
Atomic Number and Electron Count: Unveiling the Essence of Bromine
Every element in the vast universe of chemistry possesses a unique identity, defined by its atomic number. This number signifies the number of protons residing in the nucleus of an atom. Protons carry a positive charge, and their number plays a pivotal role in determining the element’s position in the periodic table.
Bromine, an element belonging to the halogen group, has an atomic number of 35. This means that every atom of bromine contains 35 protons within its bustling nucleus. The atomic number of an element is not just a random number; it’s a fundamental characteristic that dictates the chemical behavior and properties of that element.
The number of protons in an atom is intricately linked to the number of electrons, which orbit the nucleus. Electrons carry a negative charge and are equal in number to the protons in a neutral atom. This delicate balance of charges ensures the atom’s electrical neutrality.
In the case of bromine, its atomic number of 35 implies that a neutral bromine atom will have 35 electrons whirling around its nucleus. These electrons are the key players in chemical reactions, as they determine the element’s reactivity and bonding capabilities. Understanding the concept of atomic number and its relation to electron count provides a deeper insight into the enigmatic world of chemistry and the fascinating behavior of its elemental building blocks.
Electron Configuration: Unraveling the Key to Valence Electrons
Embark on a captivating journey into the realm of quantum mechanics, where the intricate arrangement of electrons within atoms holds the key to understanding their mysterious behavior.
Electron Distribution and Orbital Notation
Imagine electrons as tiny particles whirling around the atomic nucleus, each occupying a specific energy level. These energy levels are represented by orbitals, which are spatial regions where electrons are most likely to be found. Using a unique notation system, we can describe the distribution of electrons in these orbitals. Let’s take a closer look:
s orbital: Can hold up to 2 electrons
p orbital: Can hold up to 6 electrons
d orbital: Can hold up to 10 electrons
f orbital: Can hold up to 14 electrons
Bromine’s Electron Configuration
Let’s focus on bromine, an intriguing element from the halogen group. Its atomic number, 35, reveals that it has 35 electrons. By following specific rules in quantum mechanics, we can write its electron configuration:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵
Identifying Valence Electrons
The valence electrons, the most important players in chemical reactions, reside in the outermost energy level. In bromine’s case, these are the five electrons in the 4p orbital. These electrons are loosely bound and have a strong tendency to participate in chemical reactions, making bromine a highly reactive element.
Understanding Valence Electrons
Valence electrons govern the chemical properties and reactivity of elements. They play a crucial role in determining an element’s ability to form bonds with other elements, shaping the countless molecules and compounds that make up our world. By unraveling the electron configuration of atoms, we gain invaluable insights into the behavior of these fascinating building blocks of matter.