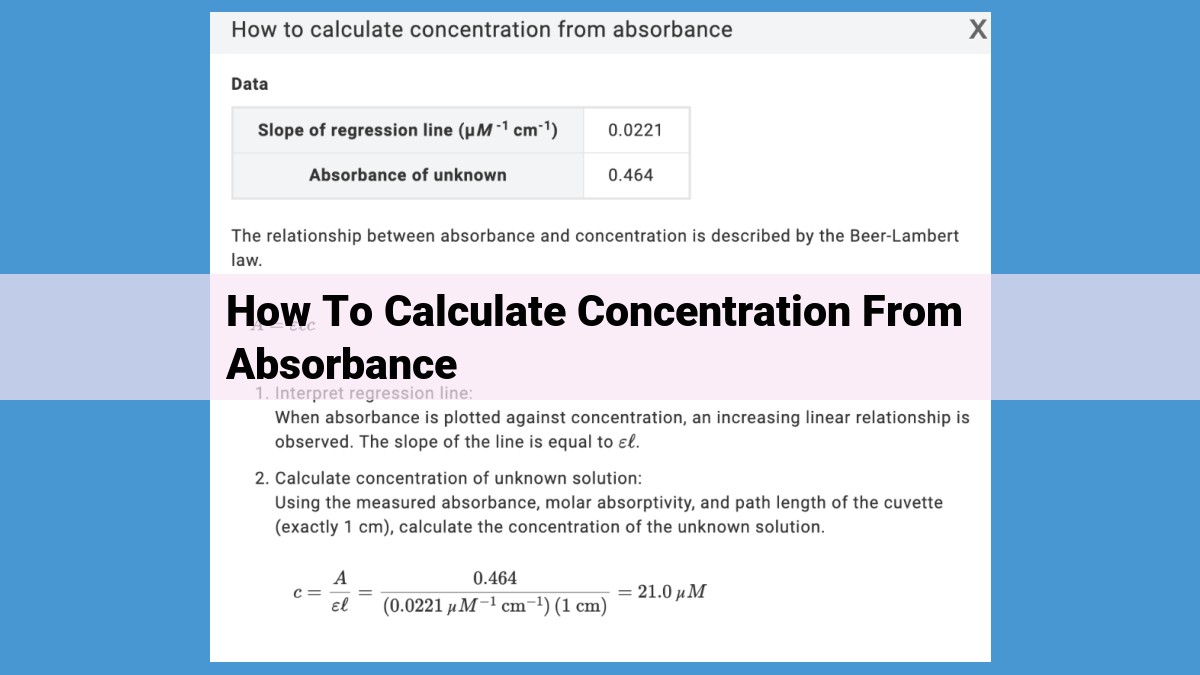
To calculate concentration from absorbance, utilize the Beer-Lambert Law (A = εbc), which establishes a direct proportionality between absorbance (A), concentration of the absorbing substance (c), path length (b), and molar absorptivity (ε), a constant specific to the substance. First, determine the path length of your sample. Then, measure the absorbance using a spectrophotometer at a specific wavelength. Divide the absorbance by the molar absorptivity to obtain the product of concentration and path length. Finally, solve for concentration by dividing this value by the path length.
Spectrophotometry: A Powerful Tool for Unlocking the Secrets of Concentration
In the realm of scientific research, where precision and accuracy are paramount, spectrophotometry emerges as an indispensable technique for unraveling the mysteries of concentration.
Spectrophotometry is a technique that utilizes the interaction of light with matter to measure the absorption of light by a substance. This absorption provides valuable insights into the concentration of the substance present in a sample. By shining light of specific wavelengths through the sample and measuring the amount of light transmitted, scientists can quantify the amount of substance present.
This ability to determine concentration makes spectrophotometry crucial in diverse fields such as analytical chemistry, biochemistry, and environmental science. From monitoring pollution levels to detecting contaminants in food and beverages, spectrophotometry provides a reliable and versatile tool for uncovering the hidden secrets of concentration.
Understanding Absorbance: A Measure of Light Absorption
In the realm of spectrophotometry, absorbance reigns as a fundamental parameter that quantifies the extent to which a substance intercepts light passing through it. This measurement plays a crucial role in determining the concentration of substances and unraveling their molecular properties.
Absorbance, denoted by the symbol A, is defined as the logarithm of the ratio of incident light intensity (I0) to transmitted light intensity (I):
A = log10(I0 / I)
This equation highlights the direct relationship between absorbance and the amount of light absorbed by the substance. The higher the absorbance, the greater the absorption of light.
Absorbance finds its mathematical expression in the renowned Beer-Lambert Law, a cornerstone of spectrophotometry. This law establishes a proportional relationship between absorbance, concentration, and the physical properties of the sample:
A = εbc
Where:
- ε is the molar absorptivity, a substance-specific constant that describes its efficiency in absorbing light.
- b is the path length, the distance light travels through the sample.
- c is the concentration, the amount of substance present in the sample.
The Beer-Lambert Law reveals the fundamental link between absorbance and concentration. By measuring absorbance at a specific wavelength, scientists can infer the concentration of the substance in the sample. This principle underpins countless applications in analytical chemistry, from quantifying pollutants to determining the purity of pharmaceuticals.
Concentration: Defining the Essence of Substance Presence
In the world of chemistry, concentration holds a central role, akin to a master storyteller unfolding a tale of substances and their realms. It captures the very essence of substance presence, painting a vivid picture of how much of a particular solute (the растворенное вещество) resides within a given solvent (the растворитель).
Just as a skilled weaver blends threads to create intricate tapestries, chemists use a diverse palette of units to express concentration. Molarity, represented by the majestic symbol “M”, stands tall as a stalwart and widely used unit. It depicts the number of moles of solute residing in a stately liter of solution. Each mole, a fundamental unit of substance quantity, embodies the immense throng of 6.022 × 10^23 particles.
Yet, the realm of concentration extends beyond the confines of molarity. Other units, such as parts per million (ppm), also grace the chemist’s lexicon. In the world of ppm, a million tiny units of solute dance gracefully within a sprawling volume of solution.
With these units as their paintbrushes, chemists orchestrate captivating chronicles of substance presence, unveiling the intricate relationships between substances and their surroundings.
Unveiling the Secrets of the Beer-Lambert Law: A Guiding Light for Spectrophotometry
In the realm of chemistry and beyond, the ability to accurately determine the concentration of substances is crucial. Enter spectrophotometry, a technique that harnesses the power of light to probe the depths of solutions and unravel their secrets. At the heart of spectrophotometry lies the Beer-Lambert Law, a cornerstone principle that governs the dance between light, matter, and concentration.
Picture a beam of light, like a tiny symphony of photons, traversing through a solution. As it encounters molecules, some photons are absorbed, their energy swallowed by the molecules’ own internal vibrations. The more molecules present, the greater the absorption of light. This interplay between light absorption and molecular concentration forms the foundation of the Beer-Lambert Law.
The law’s mathematical embodiment is a simple yet profound equation: A = εbc, where A represents the absorbance, a measure of the extent to which light is absorbed by the solution. The molar absorptivity (ε) is a substance-specific constant that reflects its eagerness to absorb light. The path length (b) is the distance traveled by the light beam through the solution, like a traveler crossing a vast desert. And finally, the enigmatic concentration (c), the very essence we seek to uncover.
The crux of the Beer-Lambert Law lies in its revelation that absorbance is directly proportional to concentration. This means that as the number of molecules in the solution increases, so too does the absorbance, like a beacon shining brighter amidst a sea of molecules. This proportional relationship makes it possible to use spectrophotometry as a precise tool for measuring concentration, opening doors to a world of analytical insights.
Path Length: The Distance Light Travels in the Sample
- Definition of path length as the distance light travels through the sample.
- Units commonly used to measure path length (e.g., centimeters).
Understanding Path Length in Spectrophotometry
Spectrophotometry, a technique used to determine the concentration of substances, involves measuring the extent to which a sample absorbs light. Path length plays a crucial role in this process, as it represents the distance that light travels through the sample.
Defining Path Length
In spectrophotometry, path length refers to the physical distance that light traverses within the sample. It is typically measured in centimeters (cm) and can vary depending on the sample holder or cuvette being used.
Importance of Path Length
Path length directly influences the absorbance of light by a sample. According to the Beer-Lambert Law, absorbance is proportional to the path length. This means that the greater the path length, the more light is absorbed by the sample.
For example, a sample with a longer path length will exhibit a higher absorbance value compared to one with a shorter path length, assuming the concentration and other factors remain unchanged. This is because the light has more opportunity to interact with the sample molecules, resulting in increased absorption.
Optimal Path Length
The choice of path length is important to ensure accurate and reproducible results. Generally, a path length of 1 cm is commonly used in spectrophotometry. This standard path length allows for reliable comparisons between samples and minimizes the risk of over- or under-estimating concentrations.
However, in certain applications, such as measuring very dilute or concentrated samples, different path lengths may be employed to optimize the signal-to-noise ratio and obtain a wider dynamic range.
Path length is a key parameter in spectrophotometric analysis. By understanding the concept of path length and its relationship to absorbance, scientists can accurately determine the concentration of substances in various samples. Careful consideration of path length optimization is essential for reliable and reproducible spectrophotometric measurements.
Molar Absorptivity: The Substance-Specific Constant in Spectrophotometry
Spectrophotometry, a powerful analytical tool, relies on the absorption of light to determine the concentration of substances. At the heart of this technique lies a crucial concept: molar absorptivity, a substance-specific constant that sheds light on the efficiency of light absorption.
Imagine light as a messenger, carrying information about the substances it encounters. As light passes through a sample, some of it interacts with the molecules, causing them to absorb energy. The extent of this absorption depends on the substance’s molecular structure and concentration. This interaction translates into a measurement we call absorbance, which quantifies the amount of light absorbed by the sample.
Molar absorptivity is the key that unlocks the relationship between absorbance and concentration. It is an intrinsic property of a substance, representing its ability to absorb light at a specific wavelength. This constant, often denoted by the symbol ε, is typically expressed in units of liters per mole per centimeter (L⋅mol−1⋅cm−1).
In the realm of spectrophotometry, molar absorptivity serves as a fingerprint for each substance. It allows us to identify and quantify even trace amounts of a substance by comparing the measured absorbance against the molar absorptivity value. This knowledge empowers scientists and researchers to delve into the molecular world, unraveling the mysteries of unknown samples.
So, when you embark on the journey of spectrophotometry, remember molar absorptivity as your guide. This substance-specific constant holds the secret to deciphering the language of light absorption, enabling you to harness the power of spectrophotometry to unlock the secrets of your samples.