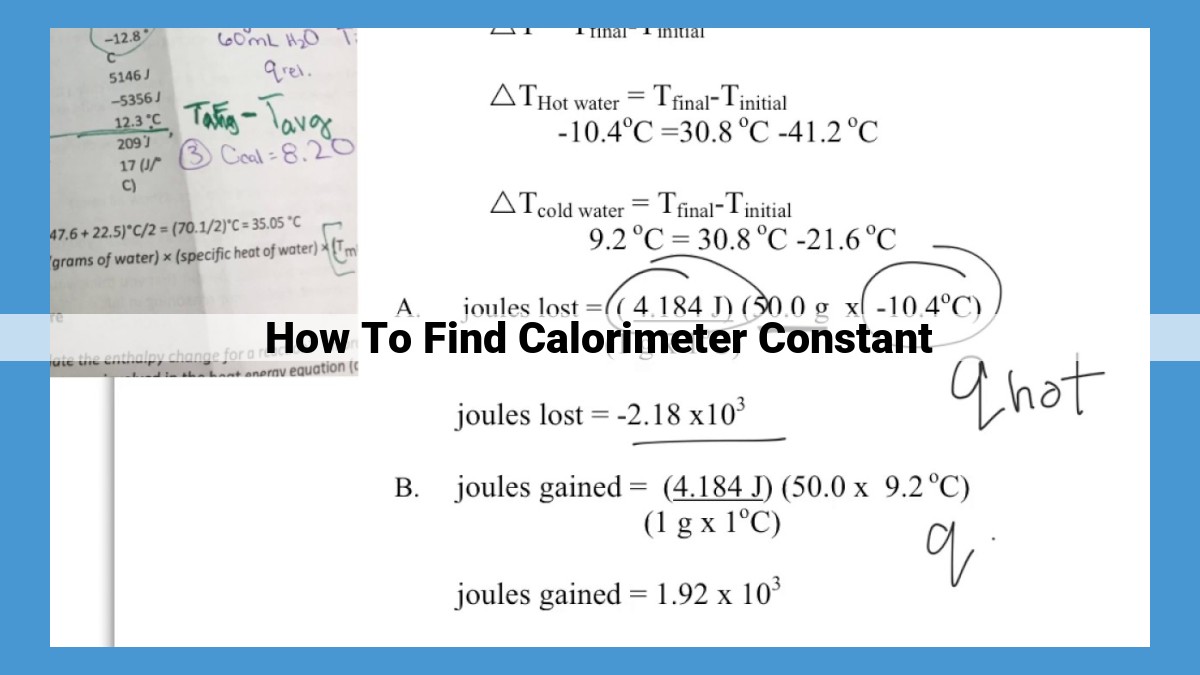
To find the calorimeter constant, determine the heat capacity of the calorimeter using the specific heat of its material and volume. Measure the temperature change of water added to the calorimeter, using its mass and specific heat. Use the formula: heat gained/lost by water = heat lost/gained by calorimeter + heat lost/gained by calorimeter * temperature change of calorimeter. Solve for the calorimeter constant, which represents the heat required to raise the calorimeter’s temperature by one degree Celsius.
Understanding Calorimeter Constant
- Definition and significance in calorimetry
- Measurement of heat capacity
Understanding the Calorimeter Constant
In the realm of calorimetry, the calorimeter constant stands as a crucial concept that hinges on the ability to measure heat capacity. Imagine yourself as a culinary artist, meticulously measuring ingredients to create a delectable dish. Similarly, in calorimetry, the calorimeter constant allows us to determine the thermal properties of materials with utmost precision.
The calorimeter constant embodies the heat capacity of the calorimeter, which encapsulates the specific heat of its material and its volume. Just as different foods have varying specific heats, calorimeters also possess unique thermal characteristics that influence their temperature response. Alongside the calorimeter’s properties, we also need to account for the temperature change of water and its mass when submerged in the calorimeter. Water, a ubiquitous substance, serves as the medium for heat exchange, and its density enables seamless conversion between volume and mass measurements. Additionally, water’s specific heat is a well-established constant value, providing a solid foundation for our calculations.
By understanding these interrelated concepts, we can embark on the process of calculating the calorimeter constant. Our formula harnesses the principles of heat transfer, equating the heat gained or lost by water to the heat lost or gained by the calorimeter, thereby incorporating the temperature change of the calorimeter.
Determining Related Concepts
In the realm of calorimetry, a fundamental concept revolves around the calorimeter constant. This value plays a pivotal role in determining the heat capacity of a calorimeter, which is crucial for precise heat transfer measurements. To unravel the significance of the calorimeter constant, we’ll delve into the intricate interplay of related concepts:
Heat Capacity of Calorimeter
The heat capacity of the calorimeter captures its intrinsic ability to store thermal energy. Two key factors influence this capacity:
-
Specific Heat of Material: Different materials possess varying abilities to absorb and retain heat. A calorimeter’s specific heat represents the amount of heat required to raise its temperature by one degree.
-
Volume of Calorimeter: The volume of the calorimeter directly impacts its heat capacity. A larger volume implies a greater capacity to absorb heat, as there is more material to store thermal energy.
Temperature Change of Water
The temperature change of the water immersed in the calorimeter is another crucial factor. Measuring the initial and final temperatures of the water allows us to calculate the heat gained or lost by the water during the experiment.
Mass of Water
Accurately determining the mass of water is essential. By measuring the water’s volume and multiplying it by its density, we can precisely quantify the mass. This information is vital for calculating the heat gained or lost by the water.
Specific Heat of Water
The specific heat of water is a well-established constant value. This value represents the amount of heat required to raise the temperature of one gram of water by one degree Celsius. This constant value is invaluable for accurately calculating the heat absorbed or released by the water.
Calculating Calorimeter Constant: A Step-by-Step Guide
In calorimetry, the calorimeter constant plays a crucial role in quantifying heat transfer and determining the specific heats of various substances. It represents the heat capacity of the calorimeter, which is essential for precise measurements.
The formula for calculating the calorimeter constant involves three main components:
-
Heat Gained/Lost by Water: This term represents the heat capacity of water and the temperature change it undergoes during the experiment.
-
Heat Lost/Gained by Calorimeter: This term encompasses the heat capacity of the calorimeter and its temperature change.
-
Temperature Change of Calorimeter: This is the change in temperature experienced by the calorimeter during the heat exchange process.
By carefully measuring these parameters, you can determine the calorimeter constant, which will enable you to accurately calculate the heat changes associated with various reactions and processes.
Determining the Calorimeter Constant: A Step-by-Step Guide
In the realm of calorimetry, understanding the calorimeter constant is paramount. It empowers us to quantify heat transfer and delve into the fascinating world of thermodynamics. So, let’s embark on an experimental journey to unveil the secrets of the calorimeter constant.
Materials:
- Calorimeter
- Thermometer
- Known mass of water
- Source of heat (e.g., hot water, electric heater)
- Insulating material
Procedure:
-
Calibrate the Thermometer: Verify the thermometer’s accuracy by measuring the temperature of a known reference point (e.g., ice water).
-
Assemble the Calorimeter: Place the known mass of water in the calorimeter and insert the thermometer. Surround the calorimeter with insulating material to minimize heat loss.
-
Measure Initial Conditions: Record the initial temperature of the water and the ambient temperature.
-
Add Heat: Carefully introduce a known amount of heat into the water using the heat source. Monitor the temperature continuously.
-
Equilibrium Temperature: Wait until the temperature stabilizes and note the final temperature.
-
Calculate the Calorimeter Constant: Use the formula: Calorimeter Constant = (Heat Gained by Water + Heat Lost by Calorimeter) / Temperature Change of Calorimeter.
Remember to convert the heat gained/lost into joules and the temperature change into kelvins for accurate calculations.
Additional Tips:
- Use a large enough mass of water to minimize temperature fluctuations.
- Stir the water gently to ensure even heat distribution.
- Record data accurately and precisely.
- Repeat the experiment multiple times to increase accuracy.
By following these steps, you can determine the calorimeter constant and gain insights into the fascinating world of heat transfer and calorimetry.
Factors Influencing Calorimeter Constant
Every scientist requires accurate and precise measurements to ensure the reliability of their findings. When it comes to measuring heat transfer, calorimeters play a crucial role. The accuracy of these measurements heavily relies on a well-calibrated calorimeter constant, which is a measure of the calorimeter’s heat capacity. However, this constant is not immune to external factors that can potentially affect its value.
Material Properties
The materials used to construct the calorimeter can significantly impact its constant. The specific heat of the materials, which represents the amount of heat required to raise the temperature of a given mass of the material by one degree, plays a significant role. A calorimeter made with a material that has a higher specific heat will require more heat to cause a measurable temperature change compared to a calorimeter made with a material that has a lower specific heat.
Temperature Range
The temperature range over which the calorimeter is used can also influence the constant. The specific heat of materials is not always constant over a wide temperature range. It may vary slightly as the temperature changes. This means that the calorimeter constant may need to be adjusted or recalibrated for different temperature ranges to ensure accurate measurements.
External Influences
External factors such as heat loss or gain from the environment can also affect the calorimeter constant. Ideally, the calorimeter should be perfectly insulated to prevent heat exchange with the surrounding environment. However, in practical situations, some heat loss is unavoidable. Factors like temperature differences between the calorimeter and its surroundings, air currents, and vibrations can contribute to heat transfer, leading to errors in the measurements. To minimize these effects, calorimeters are often placed in controlled environments or insulated with materials to reduce heat loss.
Understanding the factors that influence the calorimeter constant is essential for accurate calorimetry measurements. By carefully considering the material properties, temperature range, and external influences, scientists can ensure the reliability of their experimental results.
Unveiling the Calorimeter Constant: A Gateway to Thermal Understanding
The calorimeter constant, a pivotal element in calorimetry, is a window into the realm of energy transfer. It unveils the fundamental nature of heat capacity, a measure of a substance’s ability to absorb and store heat. By comprehending this constant, we gain insights into the thermal behavior of various substances and processes.
Specific Heat Determination: A Path to Substance Characterization
The calorimeter constant becomes instrumental in determining the specific heat of unknown substances. By carefully measuring the heat absorbed by a substance and comparing it to the temperature rise, we can deduce the substance’s specific heat capacity. This information serves as a unique fingerprint, characterizing the substance’s thermal properties.
Thermal Analysis Techniques: Unveiling Thermal Transitions
The calorimeter constant also forms the foundation of advanced thermal analysis techniques. By monitoring heat flow over time, we can identify and analyze thermal transitions such as phase changes, thermal reactions, and glass transitions. These techniques empower scientists and engineers to probe the thermal behavior of materials, unlocking their potential for various applications.
Applications in Measuring Heat Transfer and Reactions
The calorimeter constant plays a crucial role in quantifying heat transfer and reactions. Whether it’s measuring the heat of combustion of fuels or the heat released in chemical reactions, calorimeters, armed with their determined constants, provide accurate and reliable data for researchers and engineers.
As we delve into the world of calorimetry, the calorimeter constant emerges as a cornerstone. It opens up avenues for exploring the thermal properties of substances, analyzing complex thermal processes, and advancing our understanding of energy transfer. Let us embrace this concept and harness its power to unravel the mysteries of heat and temperature.