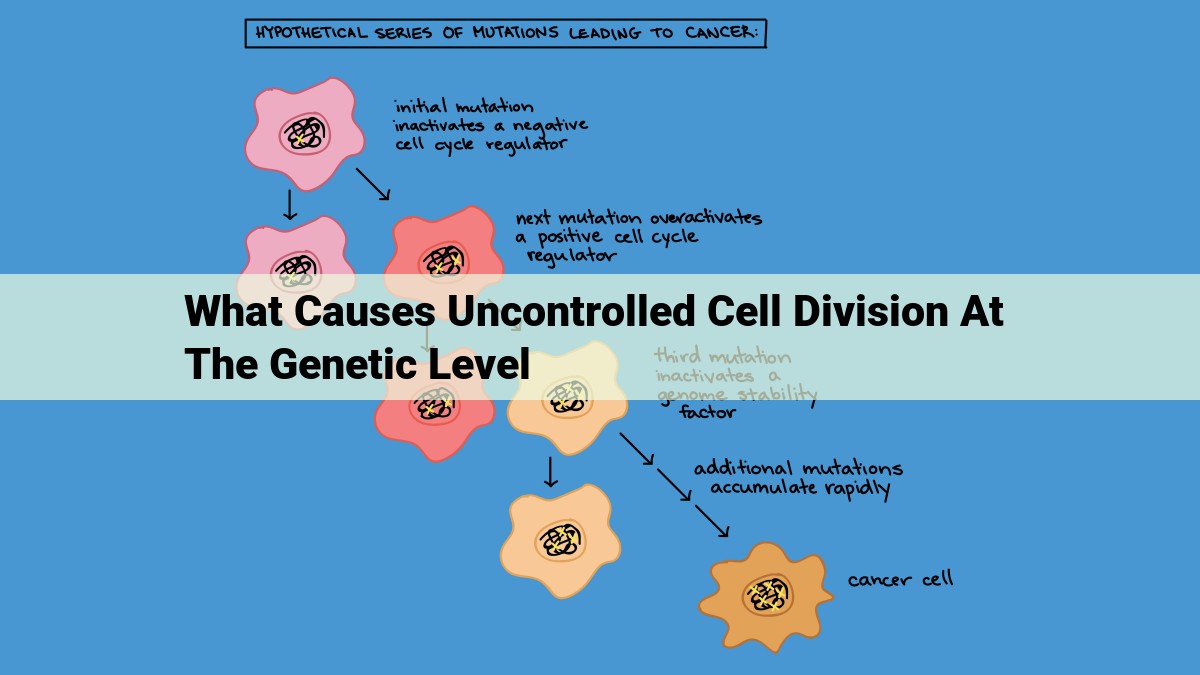
Uncontrolled cell division occurs when there are defects in genes and mechanisms that regulate cell growth and division. This can be caused by mutations in tumor suppressor genes, activation of oncogenes, defects in DNA repair, errors in cell cycle checkpoints, epigenetic changes, alterations in cell signaling pathways, viral infections, genomic instability, and dysregulated telomere maintenance.
Mutations in Tumor Suppressor Genes
- Mechanisms: Loss of heterozygosity (LOH), Inactivation of both alleles (homozygous mutations)
Unveiling the Genetic Roots of Cancer: Mutations in Tumor Suppressor Genes
Cancer, a formidable adversary, stems from a sinister dance of genetic mishaps that conspire to unleash the unchecked proliferation of cells. Among these aberrations, mutations in tumor suppressor genes play a pivotal role.
Tumor suppressor genes stand as gatekeepers of cellular integrity, silencing the unruly growth impulses of cells. However, when these protectors falter, they succumb to a cruel twist of fate: Loss of Heterozygosity (LOH) and inactivation of both alleles, leaving cells vulnerable to the insidious advance of cancer.
Loss of Heterozygosity: A Fatal Loss
LOH transpires when one of a gene’s two copies is lost, granting the remaining copy a free rein to unleash its destructive potential. This solitary gene, a lone sentinel, valiantly attempts to thwart the tumor’s march, but its efforts often prove futile.
Homozygous Mutations: Sealing the Deal
A chilling twist of fate occurs when both alleles of a tumor suppressor gene succumb to mutations, effectively silencing its protective whispers. This catastrophic event, known as homozygous mutations, plunges cells into a state of unrestrained growth, paving the way for cancer’s sinister spread.
In the murky depths of cancer’s labyrinth, mutations in tumor suppressor genes serve as a grim reminder that even the most vigilant guardians can falter under the weight of genetic mischief.
Mutations in Oncogenes
- Mechanisms: Gene amplification, Translocations
Mutations in Oncogenes: The Genesis of Cancerous Growth
In the intricate tapestry of human biology, oncogenes play a sinister role in the genesis of cancer. These genes, when mutated, embark on a path of unrestrained growth, dictating the transformation of healthy cells into malignant ones.
Gene Amplification: A Duplication of Destruction
One mechanism by which oncogenes assert their dominance is gene amplification, a process that duplicates their DNA sequences countless times. This overabundance of oncogenic DNA provides malignant cells with a formidable advantage, allowing them to outpace normal cells in the race for resources and proliferation.
Translocations: A Shuffling of Genetic Code
Another sinister tactic employed by oncogenes involves translocations, a chromosomal reshuffling that juxtaposes them with other genes. Through this strategic positioning, oncogenes can commandeer regulatory elements from their neighbors, granting them perpetual activity—a gateway to unchecked growth.
The Dire Consequences
As oncogenes amplify and translocate, their influence extends beyond their immediate surroundings, permeating the cellular landscape. They disrupt crucial pathways, unleashing a cascade of events that drive the uncontrolled proliferation, invasion, and metastasis associated with cancer. Their reign of terror erodes the integrity of our bodies, threatening our very essence.
Preventing the Onslaught
Understanding the mechanisms that fuel oncogene mutations is paramount in our quest to combat cancer. By deciphering their sinister strategies, we can develop targeted therapies that neutralize their effects and restore cellular harmony. Through this knowledge, we empower ourselves to safeguard our health and prevent the catastrophic consequences of uncontrolled growth.
Defects in DNA Repair Mechanisms: A Critical Pathway in Cancer Development
DNA Repair: The Guardian of Our Genetic Code
Our DNA is the blueprint for life. Every cell in our body relies on it to function properly. However, DNA damage is an unavoidable part of life. External factors like radiation and chemicals, as well as natural processes within cells, can create countless breaks and errors in the DNA code.
Thankfully, our cells have evolved a sophisticated system of DNA repair mechanisms to identify and fix these damages. These mechanisms work tirelessly to ensure that our genetic information remains intact.
Breaching the Defense: When DNA Repair Fails
In certain cases, these repair mechanisms can fail. This failure can be due to inherited genetic defects or acquired mutations that weaken or disable the repair proteins. When these vital defense mechanisms are compromised, defects in DNA repair arise.
These defects lead to an accumulation of DNA damage, which can have far-reaching consequences. In particular, they can disrupt the cell cycle, a tightly controlled process that ensures cells divide and grow in an orderly manner.
Consequences of DNA Repair Defects
Prolonged DNA damage can trigger a cascade of events that drive cells towards a cancerous state. For instance, unrepaired errors can result in mutations in tumor suppressor genes. These genes act as gatekeepers, preventing cells from dividing uncontrolled.
Moreover, DNA repair defects can lead to genomic instability, where the genetic material becomes prone to large-scale rearrangements and deletions. This instability can foster an environment conducive to the development of cancer.
Types of DNA Repair Mechanisms
There are several key DNA repair mechanisms that are essential for maintaining genetic integrity. Three of the most important include:
- Base Excision Repair (BER): Repairs small, damaged bases in DNA.
- Nucleotide Excision Repair (NER): Removes larger DNA lesions, such as those caused by UV radiation.
- Mismatch Repair (MMR): Corrects errors that occur during DNA replication.
Targeting DNA Repair Defects in Cancer Therapy
Defects in DNA repair mechanisms are a common feature in many types of cancer. These defects can offer potential avenues for cancer treatment. By targeting these mechanisms, researchers aim to enhance the sensitivity of cancer cells to DNA-damaging therapies, such as radiation and chemotherapy.
By understanding the mechanisms of DNA repair and the consequences of their defects, we gain valuable insights into the development of cancer. Moreover, it provides researchers with potential targets for novel and effective cancer therapies.
Errors in Cell Cycle Checkpoints: A Critical Glitch in Cellular Function
The cell cycle, a meticulously orchestrated sequence of events, is essential for the growth, division, and repair of cells. It comprises several checkpoints that monitor the cell’s progress and ensure the faithful execution of each step. However, disruptions in these checkpoints can lead to cell cycle dysregulation, often contributing to the development of cancer.
G1 Checkpoint: Assessing Growth and DNA Integrity
The G1 checkpoint, also known as the “restriction point,” is the first and most critical checkpoint. It assesses cell growth conditions and DNA integrity before the cell commits to DNA replication. If nutrients are scarce or DNA damage is detected, the G1 checkpoint halts the cell cycle, allowing time for repairs or the cell to undergo programmed cell death (apoptosis).
G2/M Checkpoint: Ensuring Accurate DNA Replication
The G2/M checkpoint, situated between DNA replication (S phase) and cell division (M phase), verifies that DNA replication has been completed without errors. It delays cell division if DNA damage is present or incompletely replicated, preventing the propagation of genetic errors.
Spindle Assembly Checkpoint: Correcting Chromosome Alignment
The spindle assembly checkpoint, activated during M phase, ensures the proper alignment of chromosomes on the spindle fibers. It prevents the cell from dividing until all chromosomes are correctly attached and positioned. When errors occur, the checkpoint arrests cell division, allowing time for corrections to be made.
Consequences of Checkpoint Errors: A Path to Malignancy
Errors in cell cycle checkpoints can have dire consequences for cell fate. Dysfunctional checkpoints may allow damaged cells to proliferate, leading to the accumulation of genetic abnormalities. This, in turn, can contribute to the development of cancer.
Cancer cells often exhibit defects in the G1 checkpoint, allowing them to bypass growth constraints and proliferate uncontrollably. Similarly, errors in the G2/M and spindle assembly checkpoints can result in aneuploidy, a condition in which cells have an abnormal number of chromosomes. Aneuploidy is a hallmark of many cancers and can promote tumor growth and metastasis.
Errors in cell cycle checkpoints are a significant contributing factor to the development of cancer. By understanding the mechanisms of these checkpoints, scientists can develop novel therapies that target their dysfunction and restore normal cell growth and division. This ultimately holds promise for the improvement of cancer treatments and patient outcomes.
Epigenetic Changes: Unraveling the Hidden Triggers of Cancer
In the intricate dance of life, our genome holds theblueprint, guiding our cells towards harmony and order. However, sometimes, this delicate symphony can be disrupted by unseen forces, leading to the insidious dance of cancer. One such force is known as epigenetics, the silent conductor that plays a pivotal role in orchestrating gene expression without altering the underlying DNA sequence.
Epigenetic changes are like invisible switches that toggle genes on or off, profoundly influencing cell behavior. Three key mechanisms underpin these changes:
DNA Methylation
DNA methylation, the addition of a methyl group to a DNA molecule, is like a molecular eraser that silences genes. When regions of DNA become highly methylated, they are effectively hidden from the cellular machinery that reads and expresses genes. This process plays a crucial role in regulating gene expression during embryonic development, but its dysregulation can contribute to cancer.
Histone Modifications
Histones are proteins that DNA wraps around, forming structures called chromatin. Chemical modifications to histones, such as the addition or removal of acetyl groups, can alter the tightness of chromatin. Acetylation loosens chromatin, making genes more accessible and promoting gene expression, while deacetylation compacts chromatin, silencing genes.
Non-coding RNAs
Non-coding RNAs, such as microRNAs (miRNAs), are short molecules that regulate gene expression by interfering with the translation of messenger RNA (mRNA). miRNAs can bind to specific mRNA molecules and block their translation, effectively inhibiting the production of proteins.
Dysregulation of these epigenetic mechanisms can lead to aberrant gene expression, disrupting cellular processes and contributing to the development of cancer. Aberrant DNA methylation patterns, abnormal histone modifications, and dysregulated miRNA expression have all been implicated in various types of cancer.
Understanding the intricate web of epigenetic changes in cancer holds tremendous promise for developing novel therapeutic strategies. By targeting these unseen forces, we may one day be able to restore the harmonious symphony of gene expression, silencing the discordant notes that lead to cancer’s deadly dance.
Alterations in Cell Signaling Pathways: Fueling the Fire of Cancer
In the intricate dance of life, cell signaling pathways act as vital messengers, orchestrating the growth, division, and death of cells. However, when these pathways go awry, they can unleash a destructive force, fueling the growth and spread of cancer.
One common culprit in cancer is the MAPK pathway. This pathway is triggered by signals from growth factors and plays a crucial role in promoting cell proliferation. Mutations in MAPK genes can cause the pathway to become overactive, leading to uncontrolled cell growth.
Another key player in cancer development is the PI3K pathway. This pathway is involved in regulating cell survival, growth, and metabolism. Mutations in PI3K genes can lead to the activation of the pathway, promoting cancer cell growth and protecting them from death.
Finally, the JAK/STAT pathway is often implicated in cancer. This pathway is triggered by cytokines, hormones, and other signaling molecules and plays a role in regulating cell division and differentiation. Mutations in JAK/STAT genes can result in the overactivation of the pathway, contributing to cancer progression.
These signaling pathways are just a few of the many that can be disrupted in cancer. By understanding the mechanisms behind these alterations, scientists can develop novel therapies to target these pathways and prevent or treat the disease.
Viral Infections: A Hidden Force in Cancer Development
Cancer is a complex disease characterized by the uncontrolled growth and proliferation of abnormal cells. While genetic mutations are often the primary culprits, viral infections play a significant role in the development of certain cancers.
Oncogenic Viruses: The Rogue Agents
Oncogenic viruses possess unique genes that can disrupt the normal functioning of human cells, leading to cancer. Some of the most well-known oncogenic viruses include:
- Human papillomavirus (HPV): Associated with cervical cancer, among others.
- Epstein-Barr virus (EBV): Linked to lymphomas, such as Burkitt lymphoma.
- Hepatitis B virus (HBV): Can contribute to the development of liver cancer.
Mechanisms of Viral Carcinogenesis
Oncogenic viruses typically disrupt cell cycle regulation, DNA repair mechanisms, or other critical cellular processes. They may:
- Insert their viral DNA into the host cell genome, altering gene expression.
- Produce proteins that interfere with checkpoint pathways, allowing damaged cells to escape natural cell death.
- Suppress the immune system, creating an environment conducive to cancer growth.
Examples of Viral-Associated Cancers
- Cervical cancer: Caused by HPV, which disrupts normal cell cycle control.
- Epstein-Barr virus-associated lymphomas: EBV infects B cells, leading to uncontrolled proliferation.
- Hepatocellular carcinoma: HBV can cause chronic liver inflammation, increasing the risk of cancer development.
Viral infections are an often-overlooked factor in cancer development. Oncogenic viruses disrupt cellular processes, create a favorable environment for cancer growth, and evade immune detection. Understanding their role is crucial for developing effective prevention and treatment strategies. By targeting these viruses, we can potentially reduce the incidence of certain types of cancer and improve patient outcomes.
Genomic Instability
- Types: Microsatellite instability (MSI), Chromosomal instability (CIN)
Genomic Instability: A Disruptive Force in Cancer Development
Within the intricate tapestry of life, our cells undergo a meticulous dance of replication and repair. However, genomic instability, like an unruly storm, disrupts this delicate balance, leaving behind a trail of mutations and chaos that can lead to the insidious development of cancer.
Microsatellite Instability: Slipping Through the Cracks
Imagine a row of identical nucleotides along DNA, like a precise line of bricks. In microsatellite instability (MSI), these bricks stumble and stutter, causing insertions or deletions that disrupt the genetic code. Certain cancers, such as colorectal and endometrial, often bear the scars of MSI, highlighting its role in tumorigenesis.
Chromosomal Instability: A Dance of Discord
Chromosomes, the guardians of our genetic blueprint, normally align and divide with precision. Chromosomal instability (CIN), however, wreaks havoc on this delicate choreography. Extra or missing chromosomes, shattered fragments, and inversions create a chaotic mosaic that can fuel cancer growth and progression.
Understanding the Triggers of Genomic Instability
The origins of genomic instability are as diverse as the cancers they seed. Defective DNA repair pathways fail to catch and mend errors, altered cell cycle checkpoints allow damaged cells to slip through, and epigenetic changes can alter gene expression, creating a breeding ground for mutations.
The Consequences of Genomic Instability
Genomic instability is a double-edged sword, slicing both for and against cancer cells. While it promotes genetic diversity that can lead to new mutations and drug resistance, it also generates chaos and dysfunction that can limit tumor growth and spread.
Harnessing Genomic Instability in the Fight Against Cancer
Despite its destructive nature, genomic instability can provide a therapeutic window in the fight against cancer. By targeting DNA repair pathways or exploiting the vulnerability of genetically unstable tumors, researchers aim to develop treatments that disarm this unruly force and restore order to the cellular landscape.
Genomic instability is a disruptive force that alters the fate of cells. Its chaotic dance can seed cancer growth, but it also presents opportunities for therapeutic intervention. By unraveling the complex mechanisms underlying genomic instability, we can unlock new strategies to tame the storm and conquer the ravages of cancer.
Dysregulated Telomere Maintenance: A Critical Factor in Cancer Development
Telomeres: The Protective Caps at the End of Our DNA
At the ends of each chromosome, there are specialized structures called telomeres. These are protective caps that safeguard the genetic information stored in our DNA during each cell division. They act like the plastic tips at the end of shoelaces, preventing the DNA strands from unraveling and becoming damaged.
Telomere Shortening: The Natural Erosion
With each cell division, telomeres gradually become shorter. This shortening is a natural process that occurs as part of normal aging. However, if telomeres shorten beyond a critical point, it can lead to cell death or the activation of cancer-promoting pathways.
Dysregulated Telomere Maintenance: A Gateway to Cancer
In cancer cells, the normal process of telomere shortening is disrupted. This can occur in two main ways:
- Telomere Shortening: In some cancers, telomeres shorten excessively, eventually reaching a critical point where the cell can no longer divide. This triggers a cellular crisis that can lead to cell death or the activation of alternative mechanisms to maintain telomere length.
- Telomerase Activation: Telomerase is an enzyme that can add nucleotides to the ends of telomeres, effectively preventing their shortening. In healthy cells, telomerase activity is tightly controlled. However, in cancer cells, telomerase activity is often high, enabling them to maintain their telomere length and continue dividing indefinitely.
Telomere Maintenance and Cancer Risk
Dysregulated telomere maintenance is a hallmark of many types of cancer. By preventing telomere shortening or activating telomerase, cancer cells can bypass the natural barriers that prevent uncontrolled cell growth. This leads to the accumulation of genetic mutations, genomic instability, and ultimately, cancer development.
Telomere maintenance is a critical aspect of cellular health and plays a crucial role in cancer biology. Dysregulation of telomere maintenance, whether through excessive shortening or unchecked telomerase activation, can lead to the development and progression of various cancers. Understanding these mechanisms is essential for developing novel therapeutic strategies to target cancer cells and improve patient outcomes.