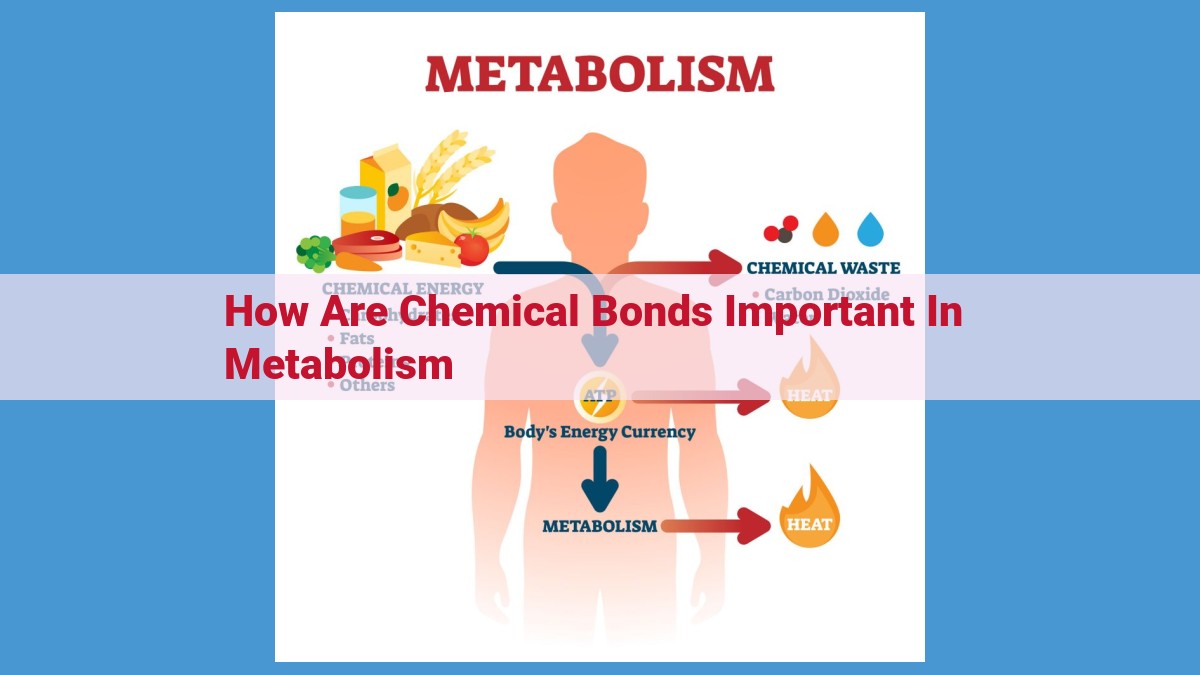
Chemical bonds, as the energy currency of metabolism, store and release free energy during metabolic reactions. They influence molecular reactivity, shape enzyme active sites, facilitating catalysis, and enable redox reactions for energy generation. Chemical bonds also serve as the building blocks of complex life structures, highlighted in supramolecular chemistry. Their understanding underpins concepts like energy metabolism, reaction kinetics, and enzyme catalysis, shaping the efficiency and regulation of metabolic processes.
Chemical Bonds: The Energy Currency of Metabolism
From the moment we wake up to the instant we drift into slumber, our bodies embark on a ceaseless symphony of chemical reactions known as metabolism. This intricate dance of molecules fuels our every move, thought, and breath. And at the heart of this metabolic ballet lie chemical bonds, the invisible force that binds atoms together and stores the energy that drives our existence.
Chemical bonds act as both energy reservoirs and energy currency in metabolic reactions. During these reactions, bonds are formed and broken, releasing or absorbing energy. Imagine a tightly wound spring. When the spring is released, its stored energy is released as it bursts back to its natural shape. Similarly, when chemical bonds are broken, the stored energy is unleashed, powering the reactions that fuel our cells.
Just as money plays a vital role in our economy, chemical bonds serve as the economic currency of metabolism. Bonds store the free energy that allows cells to synthesize complex molecules, transport nutrients, and generate electricity. Without these energy-rich bonds, our bodies would grind to a halt, lost in a sea of disconnected atoms.
Key Points:
- Chemical bonds store and release free energy during metabolic reactions.
- The energy stored in chemical bonds drives the synthesis of complex molecules, transport of nutrients, and generation of electricity within cells.
Chemical Bonds: The Building Blocks of Life
In the intricate tapestry of life, chemical bonds play an indispensable role as the invisible threads that hold the fabric of existence together. They are the architects of the molecular world, responsible for the intricate arrangement of atoms and molecules that give rise to the vibrant diversity of life forms.
Bonding: The Essence of Complexity
At the heart of chemical bonds lies the interplay of electrostatic forces. Atoms, with their positively charged nuclei and negatively charged electron clouds, exert an irresistible attraction towards one another. This delicate dance of attraction and repulsion forms the foundation of the molecular world.
Supramolecular Chemistry: Exploring Beyond Boundaries
The realm of chemical bonds extends beyond the confines of simple molecular interactions. In the captivating world of supramolecular chemistry, scientists delve into the captivating realm of intermolecular forces. These forces, though weaker than conventional chemical bonds, orchestrate the self-assembly of complex structures from individual molecules, giving rise to an array of fascinating architectures.
From self-healing materials to drug delivery systems, the applications of supramolecular chemistry are as diverse as they are transformative. By harnessing the subtle interplay of chemical bonds, researchers are unlocking new frontiers in materials science, medicine, and beyond.
In the symphony of life, chemical bonds are the master conductors, orchestrating the performance of molecules into the intricate structures that make up the living world. They are the glue that holds us together, the invisible threads that weave the fabric of our existence. As we unravel the secrets of their interactions, we gain a deeper appreciation for the profound beauty and complexity of the natural world.
Chemical Bonds: Shaping Reactivity and Metabolism
Chemical bonds, the connectors that hold atoms together, play a crucial role in the intricate tapestry of life. They are not merely inert links, but dynamic forces that shape the reactivity and efficiency of metabolic reactions.
The Type and Strength of Chemical Bonds Influence Reactivity
Different types of chemical bonds, each with its own unique characteristics, determine how molecules interact and react. Covalent bonds, with their shared electron pairs, provide stability and act as barriers to reactivity. Ionic bonds, on the other hand, involve the transfer of electrons, creating oppositely charged ions that readily interact with each other. By understanding the types of bonds present in a molecule, we can predict its chemical behavior.
Reaction Kinetics and Chemical Equilibrium in Metabolism
Chemical reactions in metabolism are governed by the principles of reaction kinetics and chemical equilibrium. Reaction kinetics describes the rate at which reactions occur, while chemical equilibrium refers to the state where forward and reverse reactions balance each other out. The strength and nature of chemical bonds influence both of these aspects.
Strong bonds, such as covalent bonds, impede reactions by requiring more energy to break. Conversely, weak bonds, like ionic bonds, facilitate reactions due to their lower energy barrier. Understanding the kinetics of metabolic reactions allows us to manipulate enzymatic reactions and optimize metabolic pathways.
Furthermore, chemical equilibrium plays a crucial role in metabolism. By establishing a balance between reactants and products, it ensures that metabolic processes proceed in a sustainable manner, preventing depletion or accumulation of specific metabolites. Thus, the type and strength of chemical bonds have a profound impact on the reactivity and efficiency of metabolic reactions, shaping the delicate balance of life’s chemical processes.
Chemical Bonds: Redox Reactions and Energy Generation
- Discuss the role of chemical bonds in facilitating electron transfer during redox reactions.
- Explain the significance of oxidative phosphorylation in energy production.
Chemical Bonds: Redox Reactions and Energy Generation
In the vibrant tapestry of life, chemical bonds serve as the threads that weave together the intricate dance of metabolism. Among these bonds, redox reactions play a pivotal role in energy generation.
Redox Reactions: A Symphony of Electron Transfer
Imagine a restaurant filled with hungry diners. Each diner represents a chemical species with varying capacities to donate or accept electrons. Redox reactions are like the waiters in this restaurant, facilitating a seamless exchange of electrons between these species.
During oxidation, a chemical species loses electrons, while in reduction, it gains electrons. This transfer of electrons is accompanied by a release or absorption of free energy. This free energy is the currency that fuels the energetic processes of metabolism.
Oxidative Phosphorylation: The Powerhouse of Energy Production
One of the most important redox reactions in metabolism is oxidative phosphorylation. This complex process takes place in the mitochondria, the powerhouses of our cells. Here, electrons from food molecules are transferred through a series of proteins called the electron transport chain.
As electrons flow down this chain, their energy is used to pump protons across the mitochondrial membrane, creating a proton gradient. This gradient is then harnessed by ATP synthase to produce ATP, the universal energy currency of cells.
The Importance of Redox Reactions
Redox reactions are essential for life. They provide the energy that powers our cells, enabling us to perform a myriad of functions, from muscle contraction to nerve transmission. By understanding the role of chemical bonds in redox reactions, we gain a deeper appreciation for the delicate balance and intricate interplay that sustains the dance of life.
Chemical Bonds: Enzyme Catalysis and Metabolic Efficiency
In the realm of metabolism, chemical bonds play a pivotal role in orchestrating the intricate dance of life. These bonds hold the key to storing and releasing free energy during countless metabolic reactions that fuel our cells. But their significance extends far beyond that. Chemical bonds are the building blocks of life, holding together the molecules that form the complex structures of our bodies and the world around us.
Enzymes, the master catalysts of metabolism, harness the power of chemical bonds to accelerate metabolic reactions with astonishing efficiency. These molecular maestros possess active sites, where specific chemical bonds create an ideal environment for reactants to interact and undergo transformations. By lowering the activation energy required for reactions, enzymes enable them to proceed at much faster rates, ensuring that the metabolic processes that sustain life run smoothly and efficiently.
To unravel the secrets of enzyme catalysis, scientists have developed sophisticated techniques such as Michaelis-Menten kinetics. This mathematical model provides a framework for understanding how enzymes interact with their substrates and how the rates of reactions are affected by factors such as substrate concentration and temperature. By studying Michaelis-Menten kinetics, researchers can gain insights into the intricate mechanisms by which enzymes orchestrate the symphony of life.
In conclusion, chemical bonds are the unsung heroes of metabolism, playing a multifaceted role in energy storage, molecular assembly, and metabolic efficiency. Enzymes, armed with the power of chemical bonds, act as the conductors of life’s symphony, ensuring that metabolic reactions proceed with precision and efficiency, fueling the intricate dance of life.