Molar enthalpy, representing the enthalpy change per mole of substance, plays a crucial role in understanding energy transformations in chemical processes. Standard molar enthalpies of formation serve as reference points for calculating molar enthalpies. Hess’s Law, involving enthalpy changes of individual steps, allows for the determination of overall enthalpy changes in reactions. Calorimetry provides experimental means to measure enthalpy changes. Molar enthalpy is essential for predicting reaction feasibility, optimizing reaction conditions, and quantifying energy content in fuels, highlighting its significance in chemical thermodynamics and practical applications.
Molar Enthalpy: The Energy Dance of Chemical Reactions
Delving into the Intriguing World of Enthalpy
Enthalpy, a crucial concept in chemistry, reveals the energy transformations that unfold within chemical reactions. It captures the combined energy of a system’s internal energy and pressure-volume work. Understanding molar enthalpy is particularly insightful, as it unveils the energy changes per mole of substance.
Molar Enthalpy: A Measure of Energy Dynamics
Molar enthalpy quantifies the energy released or absorbed during chemical reactions under constant pressure. It encapsulates the enthalpy change, which measures the difference in enthalpy between the reactants and products. By delving into molar enthalpy, scientists can comprehend the energetic interplay of chemical transformations.
Types of Enthalpy: A Spectrum of Energy Exchanges
The world of enthalpy unfolds into a spectrum of types, each reflecting a distinct energy behavior:
-
Standard Molar Enthalpy of Formation: A reference point that defines the enthalpy of a compound in its standard state.
-
Enthalpy of Combustion: The energy released when a substance burns completely with oxygen.
-
Enthalpy of Fusion: The energy absorbed when a solid transforms into a liquid.
-
Enthalpy of Vaporization: The energy required to convert a liquid into a gas.
-
Enthalpy of Reaction: The net enthalpy change associated with a specific chemical reaction.
Exothermic and Endothermic: Unveiling the Energy Flow
Chemical reactions can be classified as either exothermic or endothermic based on their energy dynamics:
-
Exothermic Reactions: Release energy into the surroundings, warming the environment.
-
Endothermic Reactions: Absorb energy from the surroundings, cooling the environment.
Harnessing the Power of Molar Enthalpy
Molar enthalpy empowers chemists with a range of insights and applications:
-
Predicting Reaction Feasibility: Determines whether reactions are spontaneous or nonspontaneous.
-
Fuel Energy Determination: Calculates the energy content of fuels, guiding efficient combustion processes.
-
Process Optimization: Optimizes reaction conditions to maximize energy efficiency and product yield.
Molar enthalpy weaves a fascinating tapestry of energy transformations, providing a deeper understanding of chemical reactions. By grasping its concepts and applications, we unlock the power to harness and manipulate energy in the world around us. The journey into the realm of enthalpy is a captivating exploration of the energetic dance of chemical systems.
Concepts Related to Enthalpy: Unraveling the Energy Dynamics of Chemical Processes
In the realm of chemistry, understanding energy changes is crucial for comprehending how chemical reactions occur and how energy flows within systems. Enthalpy, a thermodynamic property, plays a central role in quantifying these energy exchanges.
Enthalpy Change: Measuring Energy Flux under Constant Pressure
When chemical reactions occur, there’s an accompanying change in enthalpy, denoted as ΔH. Enthalpy change represents the difference in enthalpy between the initial reactants and the final products. A positive ΔH indicates an endothermic reaction, absorbing heat from the surroundings. Conversely, a negative ΔH signifies an exothermic reaction, releasing heat into the surroundings.
Molar Enthalpy: Energy per Mole of Substance
Molar enthalpy, also known as standard enthalpy of formation, is a specialized form of enthalpy that quantifies the enthalpy change per mole of a substance undergoing a chemical reaction. It’s a valuable parameter for comparing the energetic aspects of different reactions and predicting their feasibility.
For instance, the standard enthalpy of formation for water is -285.8 kJ/mol. This means that when one mole of water is formed from its constituent elements, 285.8 kJ of heat is released. Conversely, the enthalpy of formation for methane is 74.8 kJ/mol, indicating that this reaction absorbs 74.8 kJ of heat per mole of methane formed.
Types of Enthalpy: A Deeper Dive
Enthalpy, a crucial concept in chemistry, offers insights into energy changes in various processes. Among its types, the following stand out:
Standard Molar Enthalpy of Formation
This reference point measures the enthalpy change when one mole of a compound is formed from its constituent elements under standard conditions. It aids in predicting the stability of compounds and reaction spontaneity.
Enthalpy of Combustion, Fusion, Vaporization, and Reaction
a. Enthalpy of Combustion: This type quantifies the energy released when one mole of a substance burns completely in the presence of oxygen. It plays a key role in determining the energy content of fuels.
b. Enthalpy of Fusion: This value indicates the energy required to melt one mole of a solid substance into a liquid at its melting point.
c. Enthalpy of Vaporization: Similarly, this type measures the energy needed to vaporize one mole of a liquid at its boiling point.
d. Enthalpy of Reaction: This crucial concept quantifies the total energy change accompanying any chemical reaction. It aids in determining whether a reaction is exothermic (energy released) or endothermic (energy absorbed).
Exothermic and Endothermic Reactions: The Dance of Energy in Chemistry
Exothermic Reactions: A Burst of Energy
In the realm of chemical reactions, exothermic reactions take center stage as they release energy into their surroundings. Imagine a roaring bonfire, its flames dancing and crackling as they illuminate the night. This fiery display is a prime example of an exothermic reaction, where the release of energy takes the form of heat.
Endothermic Reactions: Energy Absorbers
On the opposite end of the spectrum, we find endothermic reactions. These reactions absorb energy from their surroundings, like a sponge soaking up water. Picture a block of ice slowly melting on a warm summer day. The ice absorbs energy from the air, causing its molecules to break free from their frozen state.
Characteristics of Exothermic and Endothermic Reactions
Exothermic reactions are typically characterized by:
- A decrease in enthalpy (energy) in the system
- A release of heat to the surroundings
- An increase in temperature in the system
Endothermic reactions, on the other hand, exhibit:
- An increase in enthalpy in the system
- An absorption of heat from the surroundings
- A decrease in temperature in the system
Examples of Everyday Exothermic and Endothermic Reactions
Exothermic reactions are found all around us, from the combustion of fuels to the digestion of food. Endothermic reactions also play a crucial role in daily life, such as the melting of ice and the evaporation of water.
Implications for Chemistry and Beyond
Understanding the difference between exothermic and endothermic reactions is essential for chemists and scientists alike. It allows them to predict the behavior of chemical systems, optimize reaction conditions, and harness the power of energy transformations. In industries ranging from medicine to manufacturing, the manipulation of exothermic and endothermic reactions has led to countless applications and advancements.
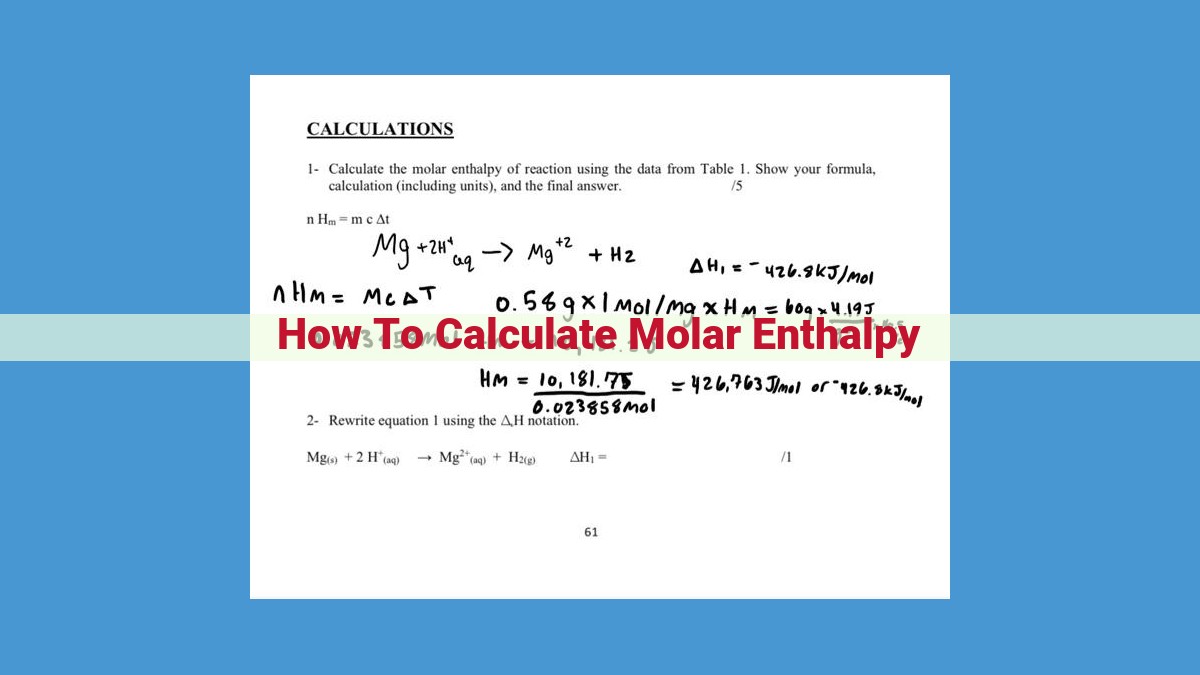
Calculating Molar Enthalpy: Unraveling the Secrets of Energy Changes
Unveiling the intricate workings of chemical reactions requires a deep understanding of molar enthalpy, a crucial parameter that quantifies energy changes in these processes. Determining molar enthalpy unlocks the key to comprehending the energetics of chemical systems.
Hess’s Law: A Guiding Light for Enthalpy Calculations
Imagine a maze of chemical reactions, each leading to a different destination. Hess’s Law is your compass in this maze, guiding you towards the net enthalpy change, the overall energy change of a reaction. By breaking down complex reactions into a series of simpler steps, Hess’s Law allows you to combine their enthalpy changes to find the total enthalpy change. It’s like assembling a puzzle, where each piece represents a reaction step, and the final image reveals the overall energy balance.
Enthalpies of Formation: The Reference Points of Enthalpy
Every substance possesses a unique enthalpy of formation, a measure of its energy content relative to its constituent elements in their standard states. These enthalpies serve as reference points, providing a baseline for calculating the energy changes in reactions. By combining the enthalpies of formation of reactants and products, you can determine the overall enthalpy change of a reaction. It’s like comparing the energy levels of different substances, and the difference tells you how much energy is released or absorbed during the reaction.
Calorimetry: Measuring Enthalpy Changes Directly
Sometimes, you can’t rely on reference data. That’s where calorimetry steps in, a technique that measures enthalpy changes directly. Imagine a controlled environment, a calorimeter, where a reaction takes place. The heat released or absorbed by the reaction is precisely measured, providing a direct measure of the enthalpy change. It’s like a thermometer for chemical reactions, quantifying the energy flow and revealing the energetics of the process.
Unveiling the Secrets of Energy Changes
Understanding molar enthalpy is the key to unlocking the secrets of energy changes in chemical systems. It empowers you to predict the feasibility of reactions, determine the energy content of fuels, and optimize reaction conditions to maximize efficiency. Whether you’re designing new materials, developing new technologies, or simply seeking a deeper understanding of the world around you, molar enthalpy is your indispensable guide. Embrace its power and unravel the mysteries of chemical reactions.
Applications of Molar Enthalpy: Unlocking the Energy Dynamics of Chemical Reactions
Molar enthalpy, a crucial thermodynamic property, plays a pivotal role in deciphering the energy transformations that accompany chemical reactions. Its applications extend far beyond mere theoretical calculations, reaching into the realm of practical applications that impact various aspects of our lives.
Understanding Energy Changes in Chemical Reactions
Chemical reactions involve the exchange or transfer of energy. By measuring the molar enthalpy change, we gain insights into the energy released or absorbed during a given reaction. This knowledge is invaluable for predicting the reaction’s feasibility and optimizing its conditions to achieve desired outcomes.
Determining Energy Content of Fuels
The combustion of fuels is a fundamental energy source for many industries and transportation systems. Molar enthalpy helps determine the energy density of fuels, providing crucial information for energy management and efficient fuel utilization. By understanding the enthalpy changes associated with combustion, we can maximize energy output while minimizing emissions.
Predicting Reaction Feasibility and Optimizing Reaction Conditions
In chemical synthesis, predicting reaction feasibility and optimizing reaction conditions is paramount. Molar enthalpy provides a thermodynamic basis for assessing reaction spontaneity and identifying the most favorable conditions for a desired chemical transformation. It enables chemists to design and optimize reactions, minimizing side reactions and maximizing product yield.
Molar enthalpy is a powerful tool that unlocks the energy dynamics of chemical systems. Its applications extend from the fundamental understanding of chemical reactions to practical applications in energy management, fuel optimization, and chemical synthesis. By harnessing the insights provided by molar enthalpy, scientists and engineers can design and optimize chemical processes, harnessing the power of energy transformations to drive innovation and progress in various fields.