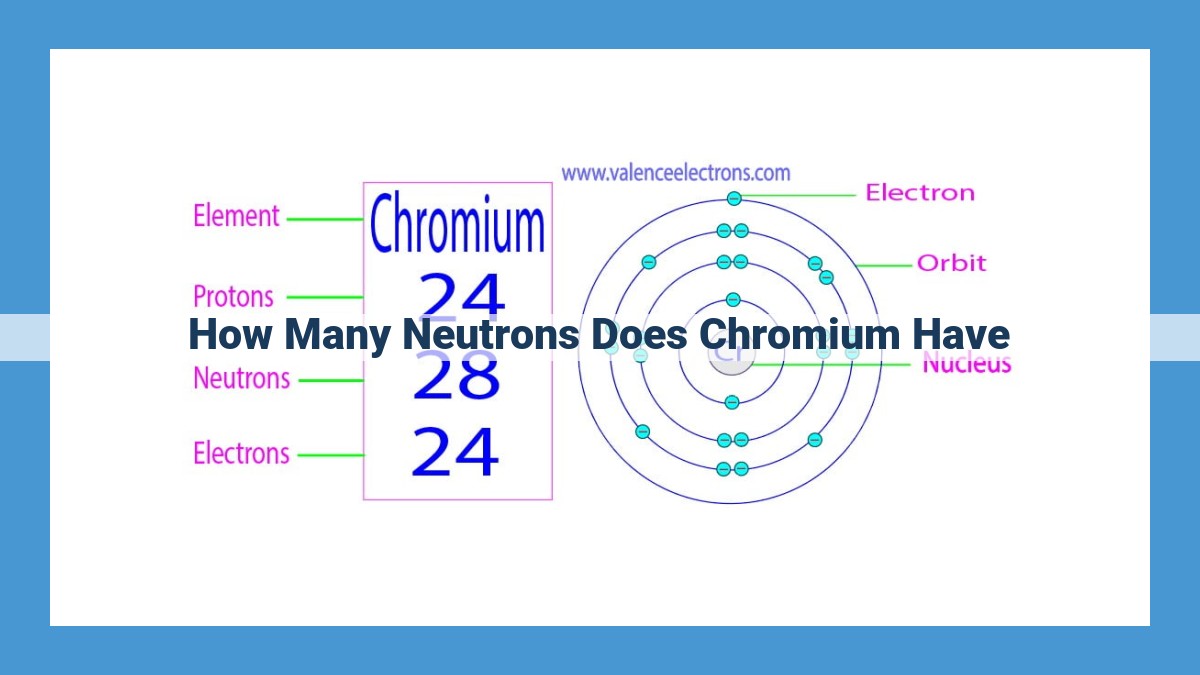
Chromium, an essential element, exhibits isotopic variations due to differing neutron counts. Chromium-52, with 28 neutrons, finds use in medical imaging. Chromium-53, with 29 neutrons, is radioactive and employed in medical diagnostics. Chromium-54, with 30 neutrons, is widely used in geology. Chromium-55, with 31 neutrons, finds applications in nuclear medicine. The most abundant isotope, chromium-56, with 32 neutrons, serves as a reference in analytical chemistry. Understanding these isotopic variations, along with their neutron counts, is crucial for comprehending chromium’s properties and applications.
- Define isotopes and their significance in understanding elemental variations.
- Highlight the importance of neutron count in defining isotopic properties.
What Are Isotopes and Why Do They Matter?
In the realm of chemistry, the smallest building blocks of matter are atoms, each composed of a nucleus and surrounding electrons. Within the nucleus, protons and neutrons reside, determining the atomic number and mass number of the atom, respectively. Variations in the number of neutrons give rise to different isotopes of an element.
Neutron Count: A Key Player in Isotopic Properties
The number of neutrons in an atom’s nucleus plays a crucial role in defining its isotopic properties. It affects the stability of the atom, its radioactive behavior, and even its physical and chemical characteristics. Understanding neutron count is essential for comprehending the diverse properties exhibited by different isotopes of an element.
Isotopes of Chromium: Unraveling the Diversity of an Essential Element
Every element in the periodic table exists in various forms known as isotopes. Isotopes are atoms of the same element with the same atomic number but different neutron counts. These differences in neutron count significantly impact isotopic properties and play a pivotal role in understanding elemental variations.
Isotopes of Chromium
Chromium, an essential metal widely used in alloys and industrial processes, exhibits a fascinating range of isotopes. Among its known isotopes are:
- Chromium-52: Characterized by 28 neutrons, it is a stable isotope that finds applications in nuclear medicine.
- Chromium-53: With 29 neutrons, it is a radioactive isotope used in medical diagnostics and cancer research.
- Chromium-54: Boasting 30 neutrons, it is a naturally abundant and stable isotope commonly employed in geological studies.
- Chromium-55: Possessing 31 neutrons, it plays a crucial role in nuclear medicine and is an isotope of interest in cancer diagnostics.
- Chromium-56: The most prevalent isotope with 32 neutrons, it serves as a stable reference isotope in analytical chemistry.
Neutron Count: A Defining Feature
The neutron count of an isotope determines several key characteristics. In the case of chromium, the neutron count:
- Influences the stability of the isotope, with isotopes with balanced neutron-to-proton ratios being more stable.
- Affects the radioactive properties of the isotope, with isotopes with an excess of neutrons often exhibiting radioactivity.
- Determines the unique applications of the isotope, as different isotopes find specialized uses in various fields ranging from medicine to geology to nuclear physics.
Unveiling the Neutron Count of Chromium-52: A Tale of Stability and Applications
Introduction:
In the vast realm of elements, isotopes stand as unique variants that shape the very essence of their parent element. Each isotope boasts a distinctive neutron count, a defining attribute that influences its properties and behavior. Among these isotopes, chromium-52 emerges as a captivating subject, its neutron count holding the key to understanding its stability and myriad applications.
Calculating the Neutron Count of Chromium-52:
Delving into the atomic structure of chromium-52, we encounter a mass number of 52. This enigmatic number represents the combined count of protons and neutrons within its nucleus. Given that the atomic number of chromium, the number of protons, is 24, we can deduce the neutron count by subtracting this value from the mass number. Thus, we uncover that chromium-52 possesses 28 neutrons.
Stability and Applications of Chromium-52:
The presence of 28 neutrons in chromium-52 bestows upon it a remarkable stability. This stability stems from the balanced interplay between the attractive strong nuclear force, which binds protons and neutrons together, and the repulsive electrostatic force between positively charged protons. As a result, chromium-52 is a non-radioactive isotope, its nucleus remaining intact over time.
This stability has propelled chromium-52 into various practical applications. Notably, it serves as a critical tracer in environmental studies, enabling scientists to track the movement of water and pollutants through ecosystems. Additionally, its high neutron capture cross-section makes it valuable in nuclear reactors as a control rod material, regulating the flow of neutrons and maintaining a stable chain reaction.
The neutron count of chromium-52, a seemingly intricate detail, unveils a profound narrative of stability and practical applications. Its 28 neutrons orchestrate a harmonious balance within its nucleus, rendering it a cornerstone of scientific investigations and industrial processes. Through this exploration, we gain a deeper appreciation for the multifaceted nature of isotopes and their pervasive influence on the world around us.
Neutron Count in Chromium-53: Exploring Its Unique Properties and Medical Significance
Neutron Count Calculation:
Like other isotopes, chromium-53’s neutron number is determined by subtracting its atomic number (24) from its mass number (53). This calculation reveals that chromium-53 contains 29 neutrons. This neutron count distinguishes it from other chromium isotopes, each with its own unique set of characteristics.
Radioactive Nature:
Unlike its more stable counterpart, chromium-56, chromium-53 is radioactive. It undergoes beta decay, a process where a neutron transforms into a proton, an electron, and an antineutrino. This decay has a half-life of 40.3 days, meaning half of the initial chromium-53 atoms will decay in that period.
Medical Applications:
Chromium-53’s radioactive properties make it valuable in various medical applications. It is used in:
- Nuclear Medicine: Chromium-53 is injected into the bloodstream to track the movement of red blood cells, providing insights into blood circulation and disorders.
- Cancer Diagnostics: The gamma rays emitted during chromium-53 decay can be used in scintillation cameras to create images of tumors and other lesions, aiding in cancer detection and diagnosis.
Chromium-53’s unique neutron count of 29 endows it with both radioactive properties and medical applications. Its ability to undergo beta decay and emit gamma rays makes it a valuable tool in nuclear medicine and cancer diagnostics. Understanding the neutron count in different chromium isotopes is crucial for comprehending their diverse properties and their implications for various fields of science and medicine.
Neutron Count in Chromium-54: A Journey into Geological Processes
Unveiling the Secrets of Chromium-54
Among the fascinating isotopes of chromium, chromium-54 stands out with its geological significance. Its neutron count, the key to its unique properties, plays a pivotal role in understanding the intricate processes that shape our Earth.
Calculating the Neutron Number
The neutron number of an isotope is the difference between its mass number (the sum of protons and neutrons) and its atomic number (the number of protons). For chromium-54, the mass number is 54 and the atomic number is 24. Subtracting the two, we find the neutron number of chromium-54 to be 30.
Abundance and Stability
Chromium-54 is the second most abundant isotope of chromium, accounting for approximately 2.36% of its natural occurrence. Its abundance is attributed to its stability, resulting from the balanced ratio of protons to neutrons. This stability has allowed chromium-54 to persist in various geological processes throughout Earth’s history.
Role in Geological Processes
The stability of chromium-54 has made it a reliable tracer in studying geological processes. Its abundance in rocks and minerals provides valuable insights into the formation and evolution of Earth’s crust. Scientists use chromium-54 to trace the movement of magma and fluids, unravel the complexities of plate tectonics, and reconstruct past climate conditions.
The neutron count of chromium-54, standing at 30, not only defines its stability but also reveals its crucial role in geological investigations. Its abundance and longevity make it an invaluable tool for unraveling the mysteries of Earth’s geological past and ongoing processes. By understanding the intricacies of chromium isotopes, we gain a deeper appreciation for the complex tapestry of our planet.
Chromium-55: A Unique Isotope for Nuclear Medicine and Cancer Diagnostics
Chromium, the versatile element, exhibits varying isotopic forms, each with distinct properties. Among these isotopes, chromium-55 stands out with its exceptional applications in nuclear medicine and cancer diagnostics.
The neutron count in an isotope determines its stability and properties. Chromium-55, with an atomic number of 24 and a mass number of 55, boasts a neutron count of 31. This neutron-rich configuration grants it unique characteristics.
In the realm of nuclear medicine, chromium-55 serves as a radiotracer. Its radioactive nature enables it to emit gamma rays, which can be detected and analyzed to study various biological processes. Physicians utilize this isotope to diagnose a wide range of conditions, including gastrointestinal disorders and kidney function.
Moreover, chromium-55 plays a pivotal role in cancer diagnostics. Its positron-emitting properties make it a valuable tool in positron emission tomography (PET) scans. In PET, chromium-55 is injected into the body, where it accumulates in tumor cells. The emitted positrons then interact with electrons, producing gamma rays that can be detected to visualize and localize tumors. This non-invasive technique has revolutionized cancer detection and management.
The neutron count in chromium-55 not only defines its stability but also empowers it with remarkable applications in nuclear medicine and cancer diagnostics. By harnessing the unique properties of this isotope, researchers and physicians can advance our understanding of biological processes and improve patient outcomes.
Exploring Chromium-56: The Stable Reference for Analytical Chemistry
Within the multifaceted realm of chromium isotopes, Chromium-56 stands as an unwavering reference point, its neutron count shaping its unique properties and indispensable role in analytical chemistry.
Neutron Count and Stability
Determining the neutron count of Chromium-56 reveals the defining characteristic that sets it apart from its isotopic counterparts. By subtracting its atomic number (24) from its mass number (56), we arrive at a neutron number of 32. This neutron count confers exceptional stability upon Chromium-56, endowing it with a remarkable resistance to radioactive decay.
Role in Analytical Chemistry
Chromium-56’s stability makes it an ideal choice as a reference isotope in analytical chemistry. In neutron activation analysis, Chromium-56 is bombarded with neutrons, causing it to transform into Chromium-57, a radioactive isotope. By measuring the emitted radiation, scientists can precisely determine the amount of chromium present in a sample.
This technique finds widespread application in diverse fields, including:
- Environmental monitoring: Assessing chromium levels in soil, water, and air
- Biological studies: Investigating chromium metabolism in living organisms
- Industrial quality control: Ensuring chromium content meets specifications in manufacturing processes
The neutron count of Chromium-56 not only defines its exceptional stability but also underpins its crucial role as a reference isotope in analytical chemistry. As a reliable and precise tool for measuring chromium levels, Chromium-56 enables scientists to uncover vital insights into the presence and distribution of this essential element across various domains.