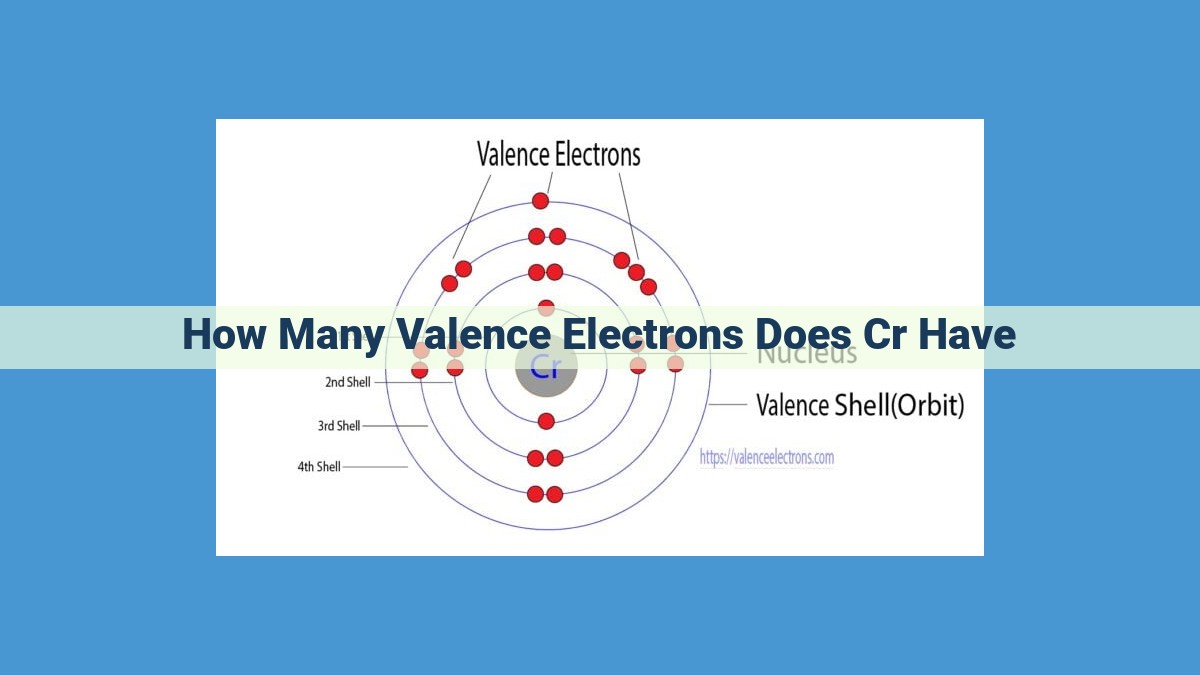
Valence electrons, found in the outermost energy level of an atom, determine an element’s chemical bonding properties. Chromium (Cr), a d-block transition metal, has an electron configuration of [Ar] 3d5 4s1. The “4s1” configuration signifies that Cr has one valence electron, occupying the 4s orbital. As a d-block element, Cr exhibits variable oxidation states due to incomplete d-orbitals, enabling it to form stable complex ions. Its unique properties make chromium valuable in alloys such as chromium steel, where its valence electrons contribute to enhanced corrosion resistance and hardness.
Unlocking the Secrets of Valence Electrons: The Key to Chemical Bonding
In the realm of chemistry, the concept of valence electrons holds immense importance, serving as the foundation for understanding how atoms interact to form molecules and compounds. Imagine atoms as miniature worlds, complete with their own nucleus and electrons orbiting around it. Valence electrons are the unsung heroes of these atomic worlds, existing in the outermost energy level, eager to participate in the dance of chemical bonding.
Why Valence Electrons Matter
Valence electrons possess a unique characteristic that sets them apart: they determine an atom’s chemical properties and its ability to form bonds with other atoms. It’s like the social butterflies of the atomic world, always seeking connections to make new friends. The number of valence electrons influences the type and strength of bonds an atom can form, and it’s this delicate balance that governs the symphony of chemical reactions.
Electron Configuration and Chromium’s Valence Electrons
Let’s take chromium as an example to explore the world of valence electrons. With an atomic number of 24, chromium (Cr) has 24 electrons that occupy specific energy levels. Using orbital notation, we can describe its electron configuration as:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹ 3d⁵
Pay close attention to the 4s¹ configuration. This indicates that chromium has one valence electron in its outermost energy level, the 4s orbital. This lone electron becomes the passport for chromium to engage in chemical bonding adventures.
The Role of Atomic Orbitals and Valence Electrons
Atomic orbitals are the designated parking spaces for electrons within an atom. They come in different shapes and sizes, with s- and p-orbitals being the most common parking zones for valence electrons. Picture these orbitals as clouds surrounding the nucleus, and valence electrons happily reside in the outermost cloud, ready to interact with their neighbors.
Chromium’s Properties and Valence Electrons
Chromium is classified as a transition metal, a group of elements known for their versatility and ability to adopt multiple oxidation states. This chameleon-like behavior is made possible by the presence of incomplete d-orbitals in their ground state. Chromium, in particular, can exhibit oxidation states of +2, +3, and +6 thanks to the flexibility of its valence electrons.
Chromium Steel: A Testament to Valence Electrons
In the world of metallurgy, chromium shines as a key ingredient in chromium steel. Its valence electrons play a crucial role in forming strong bonds with iron atoms, resulting in a material that resists corrosion and maintains its strength even under demanding conditions.
In summary, valence electrons are the unsung heroes of chemistry, dictating an atom’s chemical properties and its ability to bond with others. They are the social bridges that connect atoms, enabling the formation of molecules and compounds that shape our world.
Chromium Valence Electrons: Unlocking Chemical Bonding and Applications
Electron Configuration: A Tale of Orbits and Valence Electrons
Every atom is a microcosm of electrons orbiting a nucleus, and chromium (Cr) is no exception. Its electron configuration, written as 1s²2s²2p⁶3s²3p⁶4s¹, tells a compelling story. The “1s²2s²2p⁶3s²3p⁶” portion represents filled energy levels, while 4s¹ stands out as a lone electron in the highest energy orbital.
This valence electron is crucial because it determines how chromium interacts with other atoms. Valence electrons are the outermost electrons of an atom, and they play a pivotal role in chemical bonding. In chromium’s case, the 4s¹ valence electron indicates that it can form one bond with another atom.
Atomic Orbitals: The Dance of Electrons
Electrons don’t just float around the nucleus randomly. They occupy specific regions called atomic orbitals, which can be visualized as three-dimensional shapes. Valence electrons reside in the outermost orbitals, which for chromium are the s and p orbitals.
Chromium’s Place in the Periodic Puzzle
Chromium is a transition metal, a group of elements known for their versatility. They reside in the d-block of the periodic table, meaning they have incomplete d-orbitals in their electron configuration. This unique feature gives transition metals variable oxidation states and the ability to form stable complex ions.
Chromium exhibits multiple oxidation states, including +2, +3, and +6. This versatility makes it a valuable element in various chemical reactions.
From Valence Electrons to Chromium Steel
Chromium’s valence electron not only governs chemical bonding but also contributes to practical applications. One prime example is chromium steel, an alloy renowned for its corrosion resistance and hardness. The presence of chromium in steel enhances the material’s properties by forming strong bonds between chromium and iron atoms.
Chromium’s valence electron plays a crucial role in these bonds, determining the alloy’s strength and durability. This underscores the profound impact of valence electrons on both the fundamental properties of atoms and their practical applications.
Chromium’s Valence Electrons: The Key to Its Chemical Identity
In the tapestry of chemistry, understanding valence electrons is crucial for unraveling the intricate dance of chemical bonding. These electrons, residing in the outermost energy levels of an atom, are the primary players in forging connections with other atoms. One such element where valence electrons hold significant sway is chromium.
Chromium: A Transition Metal with a Unique Electron Configuration
With atomic number 24, chromium is a transition metal that resides in Group 6 of the periodic table. Its electron configuration, expressed in orbital notation, is [Ar] 3d5 4s1. This distinctive configuration tells the tale of chromium’s valence electrons: one electron in the 4s orbital.
Atomic Orbitals: The Electron’s Home
Imagine atomic orbitals as tiny, cloud-like spaces where electrons reside. Valence electrons occupy the orbitals with the highest energy, which in chromium’s case are the s and p orbitals. These orbitals are like the electron’s personal abodes, determining their behavior and ability to engage in chemical bonding.
Chromium’s Role in Steel: Enhanced Strength and Corrosion Resistance
Chromium’s valence electrons play a pivotal role in its practical applications. When alloyed with iron in the form of chromium steel, chromium imparts remarkable properties to this material. Its valence electrons form bonds with iron atoms, enhancing the steel’s resistance to corrosion. They also contribute to the steel’s hardness and strength, making it indispensable in various industries.
The journey into the world of valence electrons has led us to a deeper understanding of chromium’s chemical behavior and its practical significance. From its electron configuration to the formation of chromium steel, valence electrons serve as the invisible forces that shape the properties of this versatile element. By unraveling these secrets, we gain a deeper appreciation for the wonders of chemistry.
Meet Chromium: The Versatile Transition Metal with a Secret Weapon
Imagine an element with a secret power that makes it indispensable in our everyday lives. This element is chromium (Cr), a remarkable transition metal that plays a pivotal role in countless applications. Join us on an exciting journey to discover the fascinating world of chromium and its valence electrons, the key to its extraordinary properties.
Chromium, with its atomic number 24, resides in Group 6 of the periodic table. Its atomic mass of 51.9961 grams per mole and its place as a d-block element are crucial in understanding its unique characteristics. Transition metals, like chromium, possess incomplete d-orbitals in their ground state, a property that grants them exceptional chemical versatility.
Chromium’s Electron Configuration
To unravel the secrets of chromium, let’s delve into its electron configuration. Electrons, the fundamental building blocks of atoms, occupy specific energy levels called orbitals. In chromium’s case, its 4s1 configuration signifies that it has one electron in the outermost 4s orbital. This solitary valence electron holds the key to chromium’s ability to form chemical bonds with other elements.
Transition Metals: Describe the unique characteristics of transition metals, such as their variable oxidation states and ability to form stable complex ions. Mention that chromium can exhibit multiple oxidation states, including +2, +3, and +6.
Transition Metals: The Versatile Elements
In the realm of chemistry, transition metals stand out as a fascinating group of elements. Nestled within the d-block of the periodic table, these elements possess a unique set of properties that set them apart.
One of the most notable characteristics of transition metals is their variable oxidation states. This means that they can donate or accept electrons to achieve different positive or negative charge configurations. Chromium, for instance, can adopt several oxidation states, including +2, +3, and +6.
Another intriguing aspect of transition metals is their ability to form stable complex ions. These ions are typically formed when a transition metal ion bonds with ligands, which are molecules or ions that have at least one lone pair of electrons. The interaction between the metal ion and the ligands results in the formation of a coordination complex, which often exhibits interesting colors and magnetic properties.
Chromium: A Versatile Transition Metal
Chromium is a prime example of a transition metal that showcases these unique properties. With an atomic number of 24 and an electron configuration of [Ar] 3d5 4s1, chromium’s valence electron is the lone electron in the 4s orbital. This valence electron plays a crucial role in determining chromium’s chemical reactivity and ability to form bonds.
In nature, chromium is found as a hard, brittle metal with a silvery-white appearance. As a transition metal, it possesses multiple oxidation states, including +2, +3, and +6. This versatility enables chromium to form various compounds with diverse properties.
One of the most well-known applications of chromium is in the production of stainless steel. By adding chromium to steel, its corrosion resistance and hardness are significantly enhanced. This is because chromium forms a protective oxide layer on the surface of the steel, shielding it from environmental factors that cause rust and corrosion.
Transition metals, including chromium, are truly remarkable elements that exhibit a wide range of properties. Their variable oxidation states and ability to form complex ions make them indispensable in various fields, from metallurgy to catalysis. Chromium, being a versatile transition metal, is a prime example of how these elements contribute to the fascinating world of chemistry and beyond.
Valence Electrons and the Magical World of Chromium: A Tale of Chemistry and Engineering
Imagine yourself as a tiny explorer, venturing into the uncharted territories of the atom, where electrons dance around like celestial bodies. Among these electrons, there’s a special group known as valence electrons, the gatekeepers of chemical bonding.
Let’s focus our expedition on a fascinating element named chromium (Cr). With its atomic number 24, Cr holds the key to understanding the enigmatic world of d-block elements. But before we delve into its captivating properties, let’s unravel the secret of its electron configuration.
Chromium’s atomic orbitals, like tiny celestial spheres, house the precious valence electrons. The outermost orbitals, the s-orbitals, hold two electrons, while the p-orbitals play host to three more. But it’s the lone electron residing in the 4s1 configuration that holds the key to Cr’s uniqueness.
Now, let’s embark on a journey through the realm of transition metals, where Cr resides. These elements, like shape-shifting wizards, can effortlessly change their oxidation states, donning different chemical guises. Chromium’s versatility shines through its multiple oxidation states, including +2, +3, and the mighty +6.
But what makes chromium truly stand out is its membership in the d-block. Here, elements possess d-orbitals in their valence shells, like miniature energy levels that contribute to their enigmatic chemical nature. These d-orbitals play a pivotal role in the formation of stable complex ions and grant chromium its remarkable ability to form alloys and compounds.
One such marvel is chromium steel, an engineering masterpiece where Cr’s valence electrons forge unbreakable bonds with iron atoms. This alliance bestows steel with unparalleled corrosion resistance and exceptional hardness, making it the backbone of countless industries.
Chromium Steel: Discuss chromium steel as an example of the practical importance of chromium. Explain that the presence of chromium in steel enhances its corrosion resistance and hardness. Mention that the valence electrons of chromium play a role in the formation of bonds between chromium and iron atoms in steel.
Chromium: The Metallic Wonder behind Stainless Steel
In the world of chemistry, electrons hold the key to understanding the interactions between atoms. Valence electrons, in particular, play a crucial role in determining the chemical properties of an element. Let’s dive into the fascinating world of valence electrons, focusing on the versatile element chromium (Cr).
Electron Configuration
Chromium, with an atomic number of 24, has an electron configuration of [Ar]3d54s1. This tells us that its valence shell consists of one electron in the 4s orbital. This lone valence electron is of immense significance, as it determines the chemical bonding behavior of chromium.
Atomic Orbitals
Electrons occupy specific regions of space around the nucleus known as atomic orbitals. Valence electrons reside in the highest energy orbitals, which are typically the s- and p-orbitals. In chromium’s case, the valence electron occupies the 4s orbital.
Chromium: A Transition Metal
Chromium is a transition metal, located in the d-block of the periodic table. It has incomplete d-orbitals in its ground state, contributing to its unique chemical characteristics. Transition metals like chromium exhibit variable oxidation states and form stable complex ions.
Multiple Oxidation States
Chromium exhibits multiple oxidation states, including +2, +3, and +6. This versatility in oxidation states is a hallmark of transition metals and allows chromium to form diverse compounds.
D-Block Elements
The d-block elements, including chromium, have d-orbitals in their valence shells. These orbitals contribute to the chemical properties of d-block elements, influencing their bonding and reactivity.
Chromium Steel: A Practical Application
Chromium’s versatility extends beyond its chemical properties. It plays a vital role in the production of chromium steel, renowned for its corrosion resistance and hardness. The valence electrons of chromium form bonds with iron atoms in steel, enhancing its overall strength and durability.
Chromium, with its single valence electron and unique transition metal properties, is a remarkable element. Its valence electrons not only influence its chemical bonding but also contribute to the exceptional properties of chromium steel, a material that shapes our modern world. Understanding valence electrons and their significance provides a deeper appreciation for the intricate workings of chemistry.