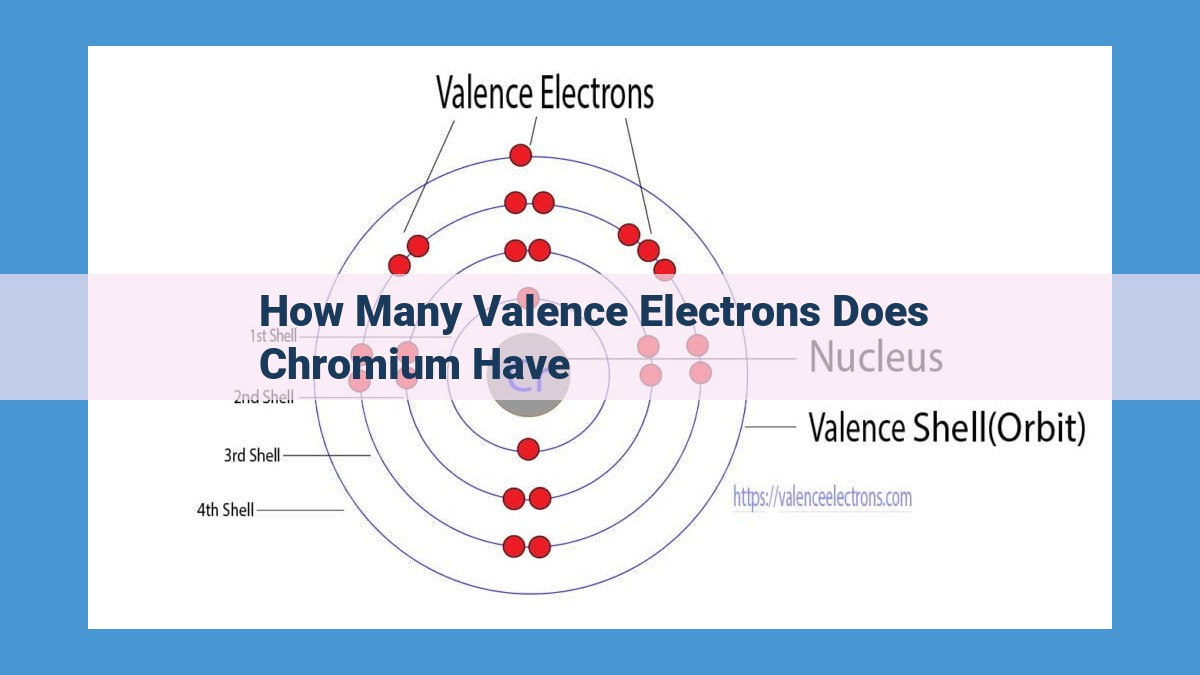
Chromium is an element with atomic number 24. Its electronic configuration is [Ar]3d54s1, which indicates that it has six valence electrons. These valence electrons are responsible for chromium’s chemical reactivity and bonding behavior. They can participate in the formation of chemical bonds, determining the element’s ability to interact with other atoms and molecules. Understanding the number of valence electrons in chromium is crucial for predicting its chemical properties and reactivity, as these electrons play a vital role in shaping its interactions with other substances.
Valence Electrons: The Gatekeepers of Chemical Interactions
In the vast tapestry of chemistry, where elements dance and form countless compounds, there lies a fundamental concept that governs their behavior: valence electrons. These are the outermost electrons in an atom’s cloak of electrons, and they wield immense power in determining an element’s chemical properties.
The Significance of Valence Electrons
Like the keys that unlock doors, valence electrons hold the secrets to an element’s bonding behavior. The number of valence electrons dictates how easily an element can gain, lose, or share electrons with others. This, in turn, determines the types of bonds it can form and the molecules it can create.
Delving into the Quantum Realm
To understand valence electrons, we must venture into the quantum realm where the world of electrons is governed by the laws of wave-particle duality. Valence electrons reside in specific energy levels around the nucleus, each with a distinct shape and energy. These energy levels are divided into orbitals, which describe the three-dimensional space where electrons are most likely to be found. Orbitals can have different shapes, such as s-orbitals (spherical), p-orbitals (dumbbell-shaped), and d-orbitals (more complex shapes).
The Dance of Hybridization
In the dance of chemistry, atoms sometimes blend their orbitals to form new entities known as hybrid orbitals. These hybrid orbitals, with their unique shapes and energies, dictate the geometry of molecules. For example, in carbon, the hybridization of s and p orbitals creates the tetrahedral shape of methane.
Blossoming into Bonds
Valence electrons are the architects of chemical bonding. They participate in covalent bonding, where they are shared between atoms, and ionic bonding, where they are transferred from one atom to another. These bonds hold atoms together, giving rise to the vast diversity of molecules that make up our world.
Chromium’s Chemical Canvas
Chromium, an element with an atomic number of 24, possesses six valence electrons. These electrons play a pivotal role in its chemistry. Chromium’s valence electrons enable it to form a wide range of compounds, from simple molecules to complex coordination complexes. Their versatility allows chromium to participate in various bonding interactions, contributing to its diverse applications in industries ranging from metallurgy to medicine.
Orbitals and Energy Levels: Understanding Electron Distribution
- Describe the concept of atomic orbitals as subdivisions of energy levels.
- Explain how these orbitals describe the three-dimensional space where electrons are most likely to be found.
Understanding Electron Distribution: Orbitals and Energy Levels
Imagine an atom as a miniature solar system, with its tiny negatively charged electrons orbiting around a positively charged nucleus. These electrons don’t just roam freely; they occupy specific regions of space called atomic orbitals.
Orbitals are like the address of electrons within an atom. Each orbital has a unique size, shape, and energy level, which determines the probability of finding an electron at that particular spot. They are analogous to roads in a city, each leading to a different destination.
The energy of an orbital increases with its distance from the nucleus. Think of it like climbing a ladder; the higher you go, the more energy required. Orbitals closest to the nucleus are filled first as electrons seek the lowest energy states.
The three-dimensional space where electrons are most likely to be found is called the orbital cloud. This cloud has a distinct shape depending on the type of orbital. The most common orbital shape is the s orbital, which resembles a sphere. Other shapes include p orbitals, which look like dumbbells, and d orbitals, which have more complex geometries.
Understanding orbitals is crucial for comprehending the chemical behavior of elements. The arrangement of electrons in orbitals determines the element’s valence electrons, which are the electrons involved in chemical bonding. By knowing the orbitals and energy levels of an element, we gain insights into its reactivity and bonding capabilities.
Hybridization: The Dance of Electrons for Molecular Geometry
In the intricate world of chemistry, electrons play a pivotal role in shaping the very structures that surround us. One fascinating concept in this realm is hybridization, a process where atomic orbitals merge, giving birth to new hybrid orbitals. These hybrid orbitals, like molecular architects, orchestrate the arrangements of atoms, dictating the shapes and characteristics of molecules.
The Birth of Hybrid Orbitals
Imagine atomic orbitals as distinct regions in space where electrons reside. When atoms embark on a chemical journey, their atomic orbitals may encounter each other, forming intimate relationships. This entwining of orbitals, known as hybridization, results in the creation of new hybrid orbitals that possess unique properties.
The Symphony of Hybrid Orbitals
The number and type of atomic orbitals involved in hybridization determine the geometry of the resulting hybrid orbitals. For instance, the hybridization of one s orbital and three p orbitals gives rise to four sp³ hybrid orbitals. These sp³ hybrid orbitals are arranged in a tetrahedral shape, pointing towards the corners of a tetrahedron.
Molecular Geometry: The Expression of Hybrid Orbitals
Hybrid orbitals exert a profound influence on molecular geometry. The arrangement of these hybrid orbitals dictates the angles between bonds and the overall shape of the molecule. For example, methane (CH₄) has a tetrahedral geometry due to the sp³ hybrid orbitals of the carbon atom. Water (H₂O), on the other hand, exhibits a bent shape because of the sp³ hybrid orbitals of oxygen.
The Dance of Hybridization in Chemical Properties
Hybridization not only governs molecular geometry but also affects other chemical properties. The type of hybridization influences the strength and polarity of chemical bonds. Hybrid orbitals with more s character are more stable and less reactive, while those with more p character are more reactive and can form stronger bonds.
Hybridization, the merging of atomic orbitals, is a captivating concept that unlocks the secrets of molecular shape and chemical properties. It provides a framework for understanding the diverse structures and functionalities of the world around us. From the tetrahedral structure of methane to the bent shape of water, hybridization orchestrates the molecular ballet that shapes our universe.
Chemical Bonding: The Symphony of Atoms
Imagine a world where materials are created by the intricate dance of atoms. This dance, known as chemical bonding, is the force that holds atoms together to form the building blocks of our universe. At the heart of this symphony of atoms lie the valence electrons.
These electrons, located in the outermost energy level of an atom, play a pivotal role in determining the atom’s reactivity and its ability to bond with other atoms. When valence electrons interact, they participate in a grand ballet of forces that give rise to the diverse array of chemical compounds we observe in nature.
There are two main types of chemical bonds: ionic bonds and covalent bonds. In ionic bonds, electrons are transferred from one atom to another, creating charged ions. In covalent bonds, electrons are shared between atoms, forming a molecular bond.
Ionic Bonds: The Transfer of Power
Imagine two atoms with very different electronegativities, a measure of their attraction for electrons. When one atom has a strong electronegativity, it can pull electrons away from another atom, creating ions. These ions, with their opposite charges, are then attracted to each other, forming an ionic bond.
Covalent Bonds: The Sharing of Secrets
Covalent bonds, on the other hand, are formed when two atoms share their valence electrons. This occurs when the atoms have similar electronegativities, resulting in a mutual attraction for the electrons. The shared electrons form a molecular bond, creating a new molecule with its unique properties.
The number of valence electrons an atom possesses dictates its bonding behavior and chemical reactivity. By understanding the dance of valence electrons, scientists can predict and manipulate the formation of new materials and compounds, unlocking the secrets of the universe’s symphony of atoms.
Valence Electrons: Unveiling Chromium’s Chemical Reactivity
In the realm of chemistry, understanding the behavior of atoms and their interactions is crucial. One fundamental concept that governs these interactions is valence electrons. Let’s delve into the fascinating world of valence electrons and explore how they influence the chemical reactivity of an element, using chromium as our exemplary case study.
Chromium: Six Valence Electrons, Boundless Possibilities
Chromium, a lustrous and versatile metal, possesses an atomic number of 24, which means it has 24 electrons orbiting its nucleus. Of these electrons, the outermost six are known as valence electrons. These six electrons occupy the highest energy level of the chromium atom, making them the most reactive and influential in determining its chemical behavior.
Valence Electrons: The Gateway to Chemical Interactions
Valence electrons are not mere passive spectators; they are the key players in forming chemical bonds, the glue that holds atoms together. Chromium, with its six valence electrons, can participate in various types of chemical bonding, including:
- Covalent Bonding: Chromium shares its valence electrons with other atoms, forming covalent bonds. These bonds are characterized by a strong attraction between atoms that share electrons.
- Ionic Bonding: In some cases, chromium can donate or accept valence electrons to form ionic bonds. When it donates three valence electrons, it becomes a positively charged chromium ion.
Chromium’s Valence Electrons: A Palette for Chemical Diversity
The number and configuration of chromium’s valence electrons dictate its chemical reactivity. For instance, chromium can exhibit different oxidation states, reflecting the number of valence electrons it gains or loses in chemical reactions. This versatility enables chromium to play diverse roles in various compounds and applications.
Chromium in Action: A Versatile Element
Chromium’s six valence electrons have propelled it into a multitude of industrial and commercial applications. Its ability to form strong covalent bonds makes it a crucial component in alloys, such as stainless steel. Additionally, its chemical reactivity enables it to serve as a catalyst in various industrial processes and as a coloring agent in paints and ceramics.
Valence electrons are the architects of chemical interactions, determining an element’s bonding behavior and reactivity. Chromium, with its six valence electrons, exemplifies the profound influence of these electrons on chemical diversity and technological advancements. Understanding the role of valence electrons is essential for unraveling the intricate tapestry of chemical reactions and the properties of elements that shape our world.