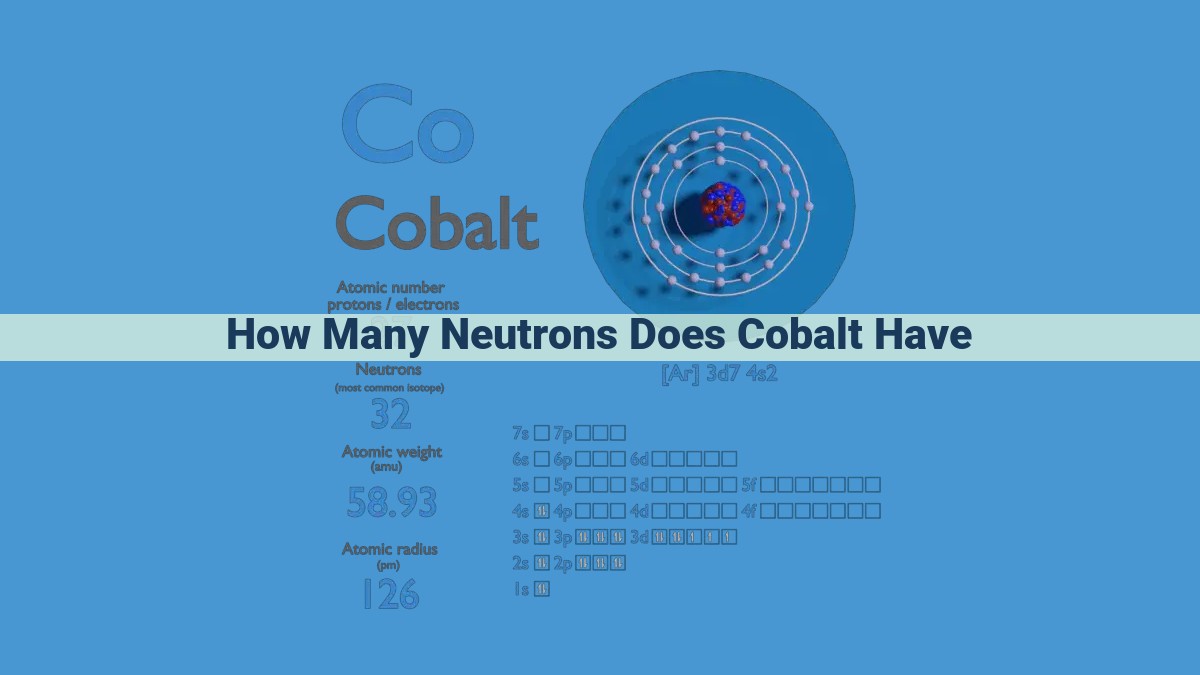
Cobalt’s atomic number of 27 indicates 27 protons in its nucleus. With a mass number of 59, the number of neutrons is calculated as 59 – 27 = 32. Therefore, each cobalt atom contains 32 neutrons, influencing its properties and applications.
Unveiling the Microscopic World: A Journey into Atomic Structure
In the realm of science, matter exists as a symphony of tiny particles called atoms, the fundamental building blocks of all substances. To unravel the mysteries of this microscopic universe, we must delve into the heart of atoms, exploring the components that define their identities.
Protons: The Mighty Nucleus
Within the heart of every atom lies its nucleus, a dense core that houses the positively charged protons. These minuscule particles, with their powerful electric charge, dictate the element to which an atom belongs. In the case of cobalt, this atomic nucleus proudly hosts 27 protons.
Electrons: The Dance of Negatives
Surrounding the nucleus is a spherical cloud of electrons, the negatively charged counterparts to protons. These ethereal particles, with their constant motion, balance the positive charge of the protons, creating an overall neutral atom. Electrons play a crucial role in determining the chemical properties of elements and their interactions with other atoms.
Neutrons: The Silent Partners
Nestled alongside protons in the nucleus are neutrons, particles that bear no electric charge. These neutral guardians, as their name suggests, contribute to the mass of the atom without influencing its charge. Neutrons act as stabilizers, preventing the positively charged protons from repelling each other and causing the nucleus to fly apart.
Understanding the Atomic Makeup of Cobalt
Atoms, the fundamental building blocks of matter, possess a fascinating internal structure that determines their unique properties. Cobalt, a transition metal with a lustrous grayish appearance, is no exception to this atomic intrigue. Let’s dive into the atomic characteristics of cobalt, exploring its atomic number, a crucial aspect that shapes its chemical behavior.
The Concept of Atomic Number: A Defining Attribute
Every element on the periodic table, including cobalt, is characterized by its atomic number. The atomic number of an element represents the number of protons found within the nucleus of its atoms. Protons, positively charged particles, reside at the heart of atoms, contributing to their overall atomic mass.
Cobalt’s Unique Atomic Number: A Chemical Fingerprint
Cobalt’s atomic number is 27, meaning each of its atoms possesses 27 protons within its nucleus. This number serves as a unique identifier for cobalt, distinguishing it from all other elements. Elements with different atomic numbers exhibit different chemical properties, hence the significance of cobalt’s specific atomic number.
Implications for the Atomic Structure and Properties of Cobalt
The atomic number of 27 plays a pivotal role in shaping cobalt’s atomic structure and properties. With 27 protons, cobalt atoms also possess 27 electrons, which orbit the nucleus in defined energy levels. This electron configuration influences cobalt’s chemical reactivity, bonding characteristics, and magnetic properties.
The atomic number of 27 is a fundamental aspect of cobalt’s atomic makeup, providing crucial insights into its chemical behavior and properties. Understanding this concept enhances our comprehension of cobalt’s significance in various industrial applications, such as the production of alloys and catalysts.
Understanding Cobalt: Delving into its Mass Number
In the world of atoms, each element is defined by its unique characteristics. One such element, cobalt, possesses a distinct mass number that plays a crucial role in understanding its properties. So, let’s embark on a journey to unravel the mystery behind cobalt’s mass number.
What is Mass Number?
In the world of nuclear physics, the concept of mass number holds significant importance. It represents the total number of protons and neutrons residing within an atom’s nucleus. The mass number serves as a signature for an element, providing vital information about its atomic structure.
Cobalt’s Mass Number: A Numerical Identity
Cobalt, symbolized by the chemical symbol Co, bears a mass number of 59. This number indicates that each cobalt atom houses a total of 59 protons and neutrons in its nucleus. The nucleus, the heart of the atom, comprises protons, which carry a positive charge, and neutrons, which remain electrically neutral.
Calculating the Number of Neutrons
To determine the number of neutrons within a cobalt atom, we employ a simple formula:
Number of Neutrons = Mass Number - Atomic Number
Since cobalt’s atomic number is 27, we can calculate the number of neutrons as follows:
Number of Neutrons = 59 - 27 = 32
Hence, each cobalt atom proudly accommodates 32 neutrons within its nucleus.
Significance of Mass Number
Understanding cobalt’s mass number is not merely academic curiosity. It holds practical importance in various scientific fields and industrial applications. The mass number influences an element’s isotopic composition, which affects its physical and chemical properties. Different isotopes of the same element possess varying numbers of neutrons, leading to variations in stability, reactivity, and other characteristics.
In the realm of metallurgy, cobalt’s mass number is crucial for alloy development and material engineering. By tailoring the isotopic composition of cobalt, scientists can create alloys with enhanced properties, such as increased strength, corrosion resistance, and thermal stability.
The mass number of cobalt, standing at 59, serves as a defining characteristic for this versatile element. It reflects the total number of protons and neutrons within its nucleus, providing valuable insights into its atomic structure and properties. Understanding cobalt’s mass number empowers scientists, researchers, and engineers to harness its potential in fields ranging from nuclear physics to materials science.
Delving into the Heart of Cobalt: Unveiling the Enigmatic Number of Neutrons
In the realm of chemistry, understanding the intricacies of atomic structure holds the key to unraveling the mysteries of the elements. Among these elements, cobalt stands out with its unique properties and diverse applications. To fully grasp the essence of cobalt, we must embark on a journey into its atomic makeup, particularly the number of neutrons it harbors.
The Foundation: Unveiling Atomic Structure
Every atom, the fundamental building block of matter, is composed of three subatomic particles: protons, electrons, and neutrons. Protons and neutrons reside in the nucleus, while electrons orbit around it. Protons carry a positive charge, electrons carry a negative charge, and neutrons, as their name suggests, carry no charge.
Colossal Cobalt and Its Atomic Fingerprint
Each element is distinguished by its atomic number, which represents the number of protons in its nucleus. Cobalt’s atomic number is 27, indicating the presence of 27 protons within its core. This unique atomic fingerprint sets cobalt apart from all other elements, defining its chemical identity.
Navigating the Mass Number Maze
Mass number is a crucial concept in atomic structure. It represents the total number of protons and neutrons within an atom’s nucleus. In the case of cobalt, its mass number is 59. This value signifies that each cobalt atom houses a total of 59 particles in its nucleus.
Unraveling the Neutron Enigma
Determining the number of neutrons in an atom is a simple yet essential step in understanding its composition. The formula for calculating this elusive number is Mass Number – Atomic Number. Applying this formula to cobalt, we embark on a mathematical adventure:
- Number of Neutrons = Mass Number – Atomic Number
- Number of Neutrons = 59 – 27
- Number of Neutrons = 32
Thus, we discover that each cobalt atom houses a staggering 32 neutrons. This information holds immense significance in understanding cobalt’s properties and its myriad applications in various industries.
Our journey into cobalt’s atomic structure has culminated in the revelation of its neutron count. This knowledge empowers us to comprehend the behavior and versatility of this remarkable element. With this newfound understanding, we can delve deeper into the world of cobalt, unlocking its potential for groundbreaking innovations and scientific advancements.