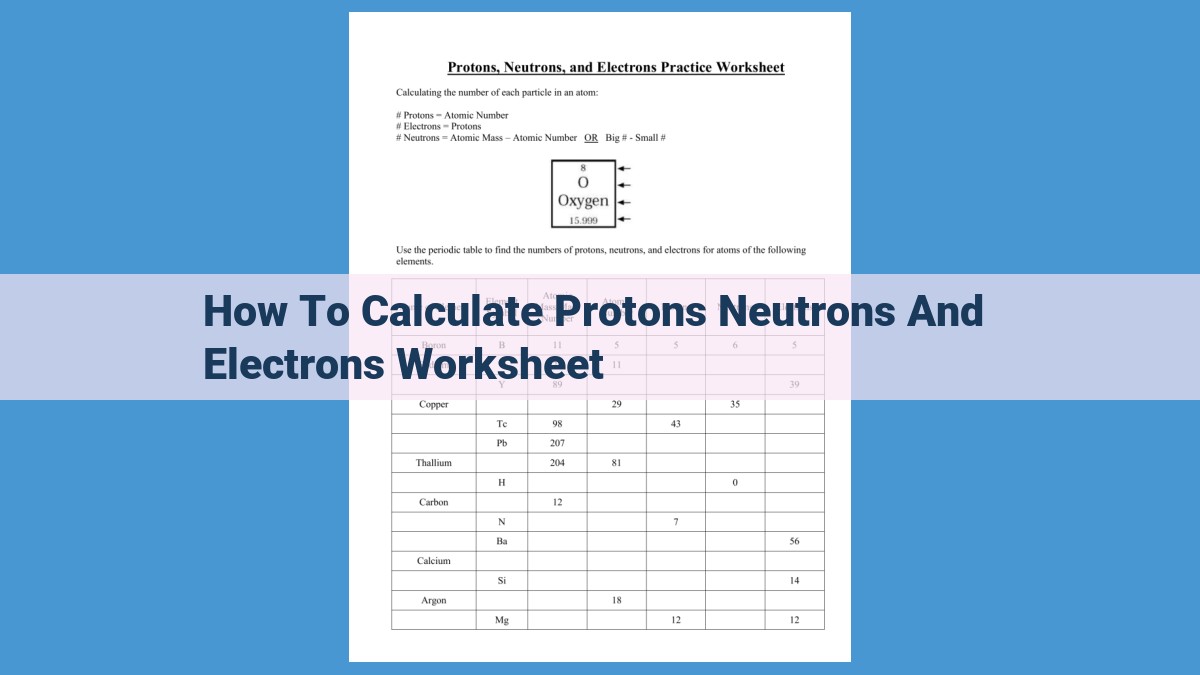
This worksheet provides a step-by-step guide to calculate the number of protons, neutrons, and electrons in an atom. It explains atomic structure, atomic number (protons), mass number (protons + neutrons), and atomic mass. The concept of isotopes (same atomic number, different mass number) is introduced, emphasizing the role of neutrons in mass but not atomic number. Protons, as positively charged particles in the nucleus, determine an element’s identity and contribute to mass. Electrons, outside the nucleus, are negatively charged and have a negligible contribution to mass.
Embark on a captivating journey into the enigmatic realm of atoms, the fundamental building blocks of all matter. Within these microscopic entities lies a tantalizing tapestry of subatomic particles, each playing a pivotal role in defining an atom’s unique characteristics.
Anatomy of an Atom: A Trio of Primary Players
At the heart of every atom reside three fundamental components: protons, neutrons, and electrons. Protons, positively charged particles, reside in the nucleus, the dense core of the atom. Their number, aptly referred to as the atomic number, dictates an element’s identity on the periodic table.
Neutrons, neutral particles that share the atomic nucleus with protons, have a profound influence on the atom’s mass but contribute nothing to its charge. Together, protons and neutrons constitute the mass number of an atom, a numerical representation of its overall mass.
Outside the nucleus, in a perpetual dance, reside the electrons, negatively charged particles. Bound by their electrostatic attraction to the positively charged nucleus, electrons determine the atom’s chemical reactivity and contribute minimally to its mass.
Unveiling the Secrets of Isotopes
Atoms of the same element can don different attire, known as isotopes. While they share the same atomic number, isotopes exhibit distinct mass numbers. This variation stems from fluctuating numbers of neutrons within the nucleus, altering the atom’s mass without affecting its fundamental identity.
The Enigmatic Neutron: A Silent Contributor to Mass
Neutrons, residing in the atomic nucleus alongside protons, are enigmatic particles. Neutral in charge, they have no direct impact on an element’s identity but contribute significantly to its mass. Their presence influences the atom’s overall stability and its radioactive properties.
Protons: The Pillars of Identity and Mass
Protons, with their positive electrical charge, form the very foundation of an element’s identity. They define an atom’s atomic number and contribute mightily to its mass. Their electrostatic attraction to the negatively charged electrons ensures the atom’s structural integrity.
Electrons: The Architects of Chemical Reactivity
Electrons, with their negative electrical charge, dance outside the nucleus in a ceaseless ballet. Their arrangement determines an element’s chemical reactivity, enabling it to form bonds with other atoms. Despite their negligible contribution to mass, electrons play a crucial role in shaping an atom’s interactions with its environment.
Join us as we delve deeper into this fascinating world of atomic structure, unraveling the secrets of matter at its most fundamental level.
**Atomic Number: The Fingerprint of Every Element**
In the vast universe of particles, atoms are the fundamental building blocks of everything that surrounds us. Within each atom lies a vital characteristic: the atomic number. It’s a unique identifier that defines an element and distinguishes it from all others.
The Proton’s Reign: Defining Atomic Number
The atomic number is the number of protons residing in an atom’s nucleus. These tiny, positively charged particles hold the key to an element’s identity. Each element has a specific and immutable atomic number that remains constant regardless of its form.
A Balancing Act: Protons and Electrons
Every atom strives for a harmonious balance of charges. The protons in the nucleus create a positive charge, while the electrons swirling around the nucleus carry a negative charge. In a neutral atom, the number of protons is equal to the number of electrons, ensuring that the overall charge is zero.
The Importance of Protons
Protons play a crucial role in determining the properties of an element. They define an element’s chemical behavior and position on the periodic table. Elements with higher atomic numbers have more protons, resulting in a greater positive charge and an increased attraction for electrons. This, in turn, influences their chemical reactivity and physical properties.
Beyond Protons: The Role of Neutrons
While neutrons in the nucleus do not directly contribute to the atomic number, they do have a significant impact on the atom’s mass. Atoms of the same element can have different numbers of neutrons, giving rise to isotopes. Isotopes share the same atomic number but differ in their mass number, which is the sum of protons and neutrons.
Mass Number: The Sum of Protons and Neutrons
In the realm of atomic structure, the mass number plays a crucial role in understanding the identity and characteristics of elements. It represents the total number of protons and neutrons found within the atom’s nucleus. This number is a critical piece of information that helps scientists classify and study the behavior of different elements.
Protons and Neutrons: Partners in the Nucleus
The nucleus, the central core of an atom, is where the action happens. Here, protons and neutrons reside, each with its unique properties. Protons carry a positive electrical charge, while neutrons remain neutral, with no electrical charge.
The number of protons in an atom determines its atomic number, which is a defining characteristic of each element. The atomic number indicates the element’s position on the periodic table and determines its chemical properties. For example, all atoms with one proton are hydrogen atoms, while all atoms with six protons are carbon atoms.
Neutrons, on the other hand, do not affect the element’s identity. They simply contribute to the atom’s mass. The mass number is calculated by adding the number of protons and neutrons in the nucleus. It is represented by the symbol A.
The Relationship between Protons and Neutrons
The relationship between protons and neutrons is crucial for understanding atomic structure and stability. In a stable atom, the number of protons and neutrons is balanced. This balance ensures that the nucleus is stable and does not undergo radioactive decay.
However, there are exceptions to this rule. Some atoms, known as isotopes, have the same number of protons but different numbers of neutrons. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons but eight neutrons. Isotopes have the same chemical properties but can differ in their physical properties, such as their mass and radioactivity.
The Significance of Mass Number
The mass number of an atom provides valuable information that is used in various scientific fields. It helps scientists determine the following:
- Identity of an element: The mass number helps identify the element, as it is related to the atomic number.
- Isotopic composition: The mass number distinguishes between different isotopes of the same element.
- Nuclear reactions: The mass number is a key factor in understanding and predicting nuclear reactions, such as radioactive decay and nuclear fission.
In summary, the mass number, as the sum of protons and neutrons in an atom’s nucleus, is a crucial characteristic that contributes to our understanding of atomic structure, element classification, and nuclear processes.
Atomic Mass
- Define atomic mass as the weighted average of isotopes
- Discuss the contributions of protons (mass) and neutrons (mass) to atomic mass
Atomic Mass: A Weighted Average of Isotopes
Understanding Atomic Mass
Atomic mass is the weighted average of the masses of an element’s different isotopes. Isotopes are atoms of the same element with the same atomic number (number of protons) but different mass numbers (number of protons + neutrons). The atomic mass of an element takes into account the abundance of each isotope in nature.
Contributions of Protons and Neutrons
Protons and neutrons both contribute to the mass of an atom. Protons carry a positive charge and are found in the nucleus of the atom, along with neutrons. Neutrons are neutral particles, meaning they have no electrical charge.
Weighted Average of Isotopes
The atomic mass of an element is calculated by multiplying the mass of each isotope by its relative abundance and then summing up the results. The relative abundance of an isotope refers to the percentage of that isotope found in a natural sample of the element.
For example, chlorine has two stable isotopes: chlorine-35 and chlorine-37. Chlorine-35 has a mass of 34.969 atomic mass units (amu) and a relative abundance of 75.77%. Chlorine-37 has a mass of 36.966 amu and a relative abundance of 24.23%.
The atomic mass of chlorine is calculated as follows:
Atomic Mass = (Mass of Cl-35 × Relative Abundance of Cl-35) + (Mass of Cl-37 × Relative Abundance of Cl-37)
Atomic Mass = (34.969 amu × 0.7577) + (36.966 amu × 0.2423)
Atomic Mass = 35.45 amu
Therefore, the atomic mass of chlorine is 35.45 amu, which reflects the weighted average of its two stable isotopes.
Isotopes: Atoms with a Twist
In the vast realm of matter, atoms are the fundamental building blocks. Each atom is a miniature universe, with an intricate structure and a unique identity. Isotopes are special members of the atomic family, atoms that share an atomic number with other members of their element but boast a different mass number.
Imagine a family of cars, all of the same make and model. However, some of these cars have different-sized engines. These variations in engine size are analogous to isotopes. Just as the cars still share a common identity despite their varying engines, isotopes have the same atomic number but different mass numbers.
The atomic number of an atom is determined by the number of protons it contains. Protons are positively charged particles that reside in the nucleus, the core of the atom. Neutrons, on the other hand, are neutral particles that also reside in the nucleus. Isotopes of the same element have the same number of protons but differ in the number of neutrons they possess.
This difference in neutron count is what accounts for the variation in mass number. The mass number of an atom is the sum of its protons and neutrons. Since isotopes have the same number of protons, their mass numbers differ based solely on the number of neutrons they contain.
Isotopes are prevalent in nature, and they play vital roles in various scientific fields, including nuclear chemistry, geology, and medicine. Understanding isotopes allows us to delve deeper into the mysteries of the atomic world and unravel the complexities of matter itself.
Neutrons: The Unsung Heroes of Atomic Structure
Nestled within the heart of every atom lies the enigmatic neutron, a fundamental building block that silently contributes to its very essence. Unlike its more charismatic counterparts, the proton and electron, the neutron operates behind the scenes, yet its presence is no less vital.
Neutrons, as their name suggests, are electrically neutral particles that reside in the atomic nucleus alongside positively charged protons. They share a common home, bound together by the strong nuclear force, an invisible power that defies the laws of electrical repulsion.
While protons and electrons play a pivotal role in defining an element’s identity and chemical behavior, neutrons contribute solely to its mass. The mass number of an atom, determined by the total number of protons and neutrons within its nucleus, is a vital characteristic that distinguishes one element from another.
Despite their lack of electrical charge, neutrons play a crucial role in stabilizing atomic nuclei. They act as a buffer, mitigating the repulsive forces between positively charged protons, ensuring that the delicate balance of the atom is maintained.
The Role of Neutrons in Isotopes
The concept of isotopes further highlights the importance of neutrons. Isotopes are atoms of the same element that possess different neutron counts. While they share identical atomic numbers, their mass numbers vary, giving rise to the phenomenon of isotopic variations.
For instance, carbon-12, carbon-13, and carbon-14 are three isotopes of carbon. They all have six protons and six electrons, but they differ in the number of neutrons. Carbon-12 has six neutrons, carbon-13 has seven, and carbon-14 has eight. This variation in neutron count affects the overall mass of the isotopes, making carbon-14 heavier than the other two.
The Significance of Neutrons in Nuclear Reactions
Beyond their atomic role, neutrons also play a pivotal part in nuclear reactions. When a neutron is absorbed by an atom’s nucleus, it can trigger a series of reactions that release tremendous amounts of energy. This process, known as nuclear fission, is the basis of nuclear power plants and nuclear weapons.
Neutrons also participate in nuclear fusion, a process in which two atoms combine to form a heavier atom. In this reaction, neutrons act as intermediaries, facilitating the merger of the atomic nuclei and releasing even more energy than in nuclear fission.
Though often overshadowed by their more visible counterparts, neutrons are indispensable to the structure and behavior of atoms. From stabilizing atomic nuclei to influencing isotopic variations and driving nuclear reactions, neutrons quietly shape the very fabric of our world. Without them, atoms would be unstable and the elements as we know them would cease to exist.
Protons: The Cornerstones of Identity
In the bustling metropolis of an atom, protons reign supreme as the positively charged particles that reside within the heart of the nucleus. These tiny subatomic wonders play a pivotal role in determining an element’s unique identity.
The number of protons within an atom’s nucleus defines its atomic number, a fundamental property that dictates its elemental classification. This number is like a cosmic barcode, assigning each element its own distinct place in the periodic table.
Furthermore, protons make a significant contribution to the overall mass of an atom. Although they do not possess the same heft as neutrons, their positive charge and compact size give them a palpable impact.
Electrons: The Negatively Charged Particles Outside the Nucleus
Electrons, the tiny, negatively charged particles that orbit the atomic nucleus, play a crucial role in the identity and behavior of atoms. These subatomic particles are what give atoms their chemical properties and determine their place on the periodic table.
Electrons have a negligible mass compared to protons and neutrons, the other two subatomic particles found in the nucleus. Consequently, they do not significantly contribute to an atom’s mass number. However, their number is directly related to an element’s atomic number, which identifies the element and determines its chemical characteristics.
For instance, all atoms with six electrons are carbon atoms, regardless of the number of protons or neutrons they possess. This relationship between the number of electrons and atomic number is essential for understanding the behavior of elements in chemical reactions.
Electrons are arranged in shells and subshells around the nucleus, with each shell holding a specific number of electrons. The innermost shell can hold up to two electrons, while the next shell can accommodate up to eight electrons, and so on. The arrangement of electrons in these shells influences the atom’s chemical reactivity and its ability to form bonds with other atoms.
In summary, electrons, despite their seemingly insignificant mass, are vital to the identity and behavior of atoms. Their number determines an element’s atomic number, their arrangement affects chemical reactivity, and they facilitate the formation of bonds between atoms, shaping the vast array of molecules and substances that make up our world.