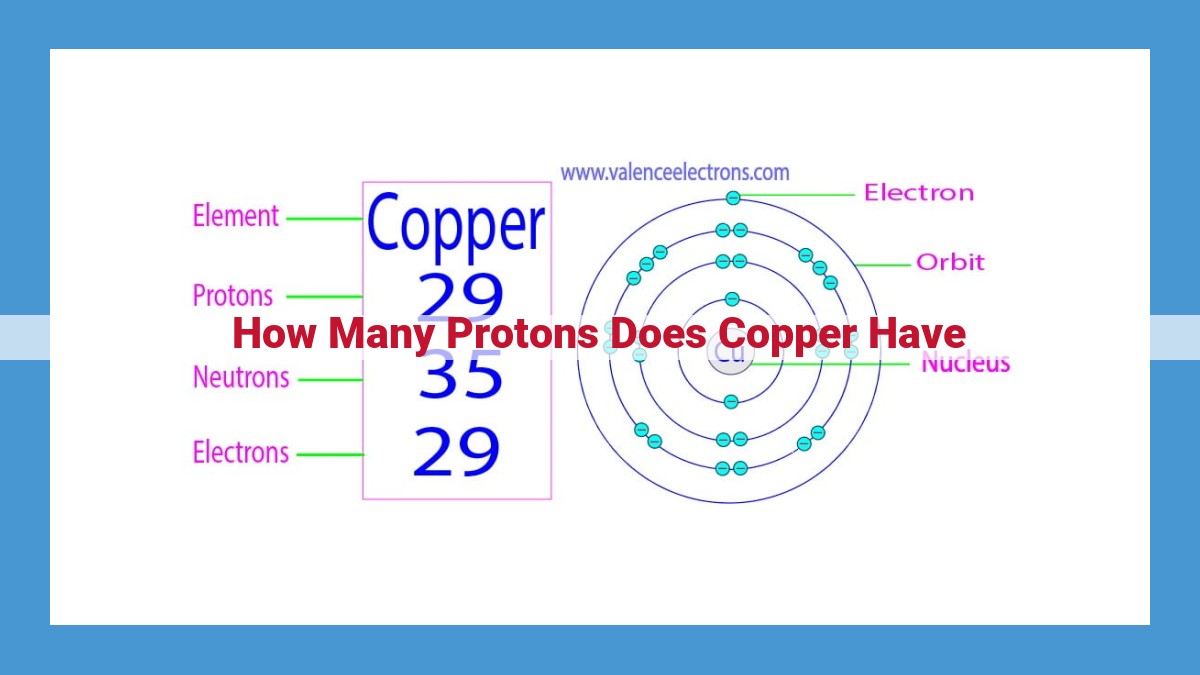
Copper, with an atomic number of 29, has a total of 29 protons within each of its atoms. The presence of these 29 protons defines copper as an element and grants its nucleus a positive charge, influencing its physical and chemical behavior. Copper’s atomic number plays a crucial role in determining its properties and distinguishing it from other elements.
Understanding Atomic Number
- Define atomic number as the number of protons in an atom.
- Explain that protons are subatomic particles with a positive charge located in the nucleus.
Understanding the Atomic Number: The Identity of Atoms
In the realm of the infinitely small, atoms, the building blocks of matter, possess a unique characteristic known as the atomic number. This crucial value defines the identity of an element and unravels the secrets of its atomic structure.
Protons: The Heart of the Atom
The atomic number represents the count of protons, the subatomic particles that reside in the heart of an atom, called the nucleus. Protons carry a fundamental positive charge and play a pivotal role in shaping an element’s behavior.
Protons and Atomic Number: An Unbreakable Bond
The number of protons in an atom is an immutable property. It defines the element to which the atom belongs. Changing this number would fundamentally alter the element’s identity, transforming it into a different one entirely.
Copper: A Transition Metal with a Unique Atomic Number
Copper, a reddish-brown metal, belongs to the family of transition elements. Its atomic number is 29, a defining characteristic that holds the key to understanding its atomic structure and properties.
Protons and Atomic Number
The atomic number of an element is a defining characteristic that sets it apart from all other elements. It is like the unique fingerprint of an element, determining its identity and dictating its behavior in the world of chemistry. The atomic number is a number that represents the number of protons found in the nucleus of each atom of that element.
Protons are tiny, positively charged subatomic particles that reside in the heart of an atom, nestled within its nucleus. They play a crucial role in shaping the identity of an element. The number of protons in an atom cannot be changed without fundamentally altering the element’s very nature. In other words, changing the number of protons transforms the atom into a different element altogether.
This concept is fundamental to understanding the behavior of elements in chemical reactions. The number of protons in an atom dictates the element’s chemical properties, such as its reactivity and its tendency to form bonds with other elements. It’s like the blueprint that governs the element’s interactions with the world around it. The atomic number is a constant for a given element, a cornerstone of chemistry that allows us to understand and predict the behavior of matter.
Understanding the Significance of Atomic Number in Copper
Imagine a microscopic world within the depths of matter, where atoms—the fundamental building blocks of everything—reign supreme. Each atom is like a miniature solar system, with a nucleus at its center and electrons orbiting it. The atomic number, an essential characteristic that distinguishes one element from another, is determined by the number of protons residing in the nucleus.
Copper: A Noble Metal and Transition Element
Copper, a malleable and ductile metal, has earned its place among the transition elements. As a transition metal, copper possesses unique properties that render it invaluable for various applications, from electrical wiring to jewelry. Its resilience and ability to withstand tarnishing have made it a trusted material for centuries.
The atomic number of copper, as we shall discover, plays a crucial role in unlocking the secrets of this remarkable element.
The Significance of Copper’s Atomic Number
Delving into the atomic structure of copper, we find that its nucleus contains not just protons but also neutrons, subatomic particles without any electrical charge. For copper, the atomic number is 29, indicating that each copper atom contains 29 protons. This number is a fundamental constant that defines copper’s identity as an element.
Just as a unique fingerprint identifies an individual, the atomic number serves as a distinct marker for each element. Changing the atomic number would alter the very nature of the element, transforming it into something entirely different.
In the case of copper, its 29 protons bestow upon the nucleus a net positive charge, making it an electropositive element. This positive charge exerts a magnetic force, which contributes to copper’s various physical and chemical properties.
How Many Protons Does Copper Have?
As we embark on a captivating journey into the realm of atomic science, let’s unravel the secrets of copper, a remarkable element with a fascinating story to tell.
Copper, a metal renowned for its remarkable versatility, holds a unique place in the periodic table. To fully grasp its essence, we must delve into the very heart of its atomic structure, where tiny subatomic particles known as protons reside.
Protons: The Cornerstone of Atomic Identity
Imagine a tiny nucleus, the control center of every atom. Within this nuclear sanctuary, protons, positively charged particles, play a pivotal role. They determine an element’s identity, like a molecular fingerprint that distinguishes one element from another.
Copper’s Atomic Number: 29
Now, let’s unveil the atomic number of copper, which holds the key to unlocking its unique properties. The atomic number is simply the number of protons within an atom’s nucleus, like a numerical passport that defines each element. Remarkably, the atomic number of copper is 29, a number that sets it apart from all other elements.
Each Copper Atom: A Proton Powerhouse
Every single copper atom carries this distinctive mark. It houses 29 protons in its nucleus, like tiny magnets creating a positive force within the atom. This proton count is what makes copper uniquely copper, giving it its characteristic nature that we observe in the world around us.
Implications of Proton Count on Copper’s Behavior
Copper’s atomic number, 29, significantly influences its physical and chemical properties. Its 29 protons grant its nucleus a positive net charge, which has profound effects on its interactions with other atoms and molecules.
The abundance of protons renders copper electrically conductive. Protons, being positively charged, allow the flow of electrons, enabling copper to conduct electricity efficiently. This property makes copper a crucial component in electrical wiring and electronics.
The proton count also affects copper’s magnetic susceptibility. The spinning protons create a magnetic field, making copper weakly responsive to magnetic forces. This susceptibility allows copper to be used in magnetic resonance imaging (MRI) and other medical applications.
In chemical reactions, the proton count dictates copper’s stability and reactivity. The strong positive charge of the nucleus firmly holds electrons, making copper relatively stable. This stability makes copper resistant to corrosion and oxidation, contributing to its widespread use in architecture and construction.
However, under specific conditions, copper can exhibit chemical reactivity. Its tendency to gain or lose electrons depends on the chemical environment and the number of valence electrons it possesses. Copper can form stable compounds with other elements, including oxygen, sulfur, and chlorine, highlighting its versatile chemical behavior.