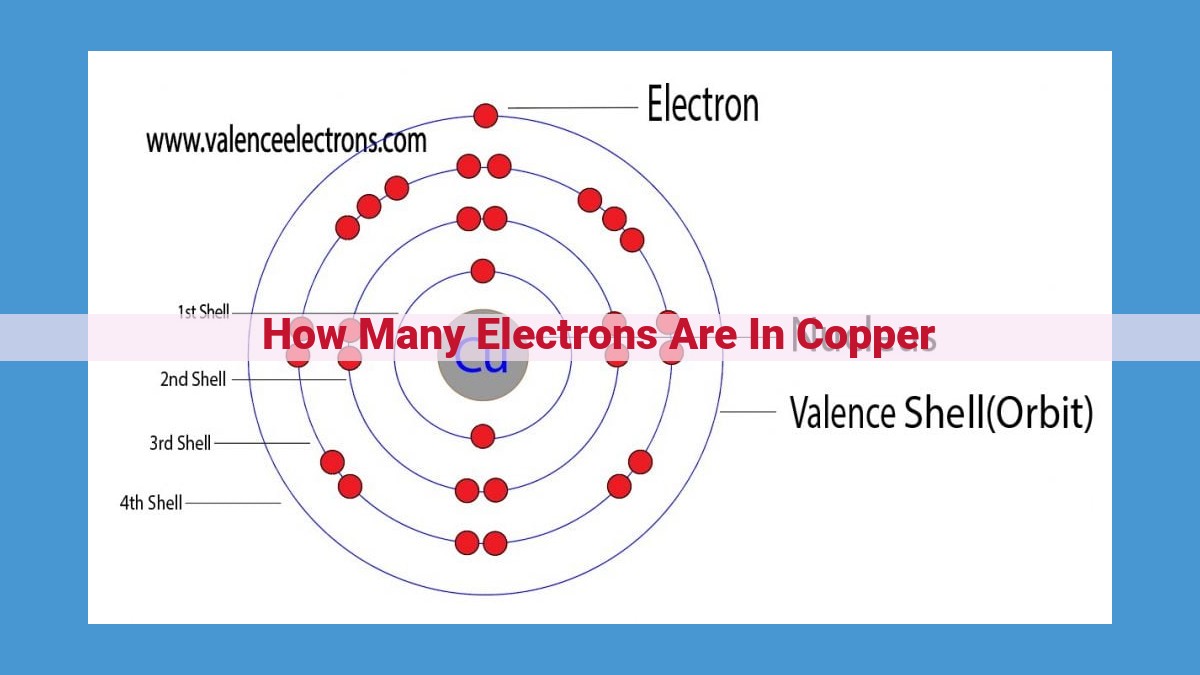
- Copper, an essential transition metal, has an atomic number of 29, signifying the presence of 29 protons and, thus, 29 electrons.
The Captivating Tale of Copper’s Atomic Structure
In the vast realm of elements, let’s embark on a fascinating journey to uncover the atomic secrets of copper, a transition metal that has captivated civilizations for centuries. From its indispensable role in electronics to its crucial involvement in biological processes, understanding copper’s atomic structure is key to comprehending its remarkable properties and applications.
Prologue: A Transition Metal with a Multifaceted Legacy
Copper stands as a shining example of a transition metal, possessing both malleability and ductility, attributes that have made it a valuable material since the dawn of human ingenuity. Its warm, reddish-orange hue has captivated artists and artisans, while its exceptional electrical conductivity has revolutionized the realm of electronics.
Atomic Number: The Foundation of Identity
Every element’s atomic number serves as its unique fingerprint, dictating the number of protons residing in its nucleus. For copper, this atomic number reads 29, indicating the presence of 29 protons. This fundamental attribute distinguishes copper from all other elements in the periodic table.
Neutron Variations: The Story of Copper Isotopes
Atoms of the same element can exhibit different neutron counts, giving rise to isotopes. Copper boasts an array of stable isotopes, each with a unique neutron number. The most prevalent among these is ⁶³Cu, possessing 34 neutrons. These isotopic variations subtly influence copper’s physical properties, shaping its behavior in various applications.
Electron Count: A Balancing Act
The harmony of an atom’s structure demands a delicate balance between the number of protons and electrons. In copper’s case, the 29 protons attract an equal number of electrons, maintaining a neutral charge. These electrons occupy specific energy levels, forming the basis of copper’s electron configuration.
Electron Configuration: A Blueprint for Orbital Distribution
Electron configuration paints a vivid picture of how electrons are distributed within an atom’s orbitals. For copper, this configuration reads [Ar] 3d¹⁰ 4s¹. This notation reveals the presence of 10 electrons in the 3d orbitals, one electron in the 4s orbital, and a filled set of electrons in the argon core. This arrangement underpins copper’s chemical reactivity and behavior.
Atomic Number: Unraveling the Essence of Copper’s Identity
In the realm of chemistry, atomic number holds immense significance, akin to an element’s unique fingerprint. It embodies the number of protons within an atom’s nucleus, defining its very essence. For the enigmatic metal copper, this atomic number stands at 29, a crucial piece of information that unlocks the door to understanding its remarkable properties.
The concept of atomic number is deeply intertwined with the concept of protons. These positively charged particles reside within the nucleus of an atom, forming its core. Each element possesses its own distinct atomic number, which directly corresponds to the number of protons it harbors. For copper, this number is 29, indicating that every copper atom contains 29 protons.
This seemingly simple number holds profound implications for copper’s behavior and characteristics. It governs the chemical reactions in which copper participates, determines its physical properties, and shapes its role in various industrial and biological processes. By comprehending the significance of its atomic number, we gain invaluable insights into the very nature of copper and its impact on our world.
Neutron Variations: Copper’s Isotopic Diversity
Copper, an indispensable transition metal, plays a crucial role in our world, from electronics to construction. But what lies beneath its metallic surface? Let’s delve into the fascinating realm of copper’s isotopes.
Understanding Isotopes: A Tale of Neutron Counts
In the atomic world, isotopes are like siblings of the same element, sharing the same atomic number but differing in the number of neutrons in their nuclei. Neutrons, neutral particles that reside in the nucleus alongside protons, contribute to the overall mass of an atom.
Copper’s Isotopic Family
Copper, with an atomic number of 29, has a family of stable isotopes. Each isotope sports a different neutron count, giving rise to unique properties. The most prevalent isotope, accounting for over 69% of naturally occurring copper, is ⁶³Cu. This isotope boasts 34 neutrons, giving it an atomic mass of 62.93.
⁶³Cu: The Workhorse Isotope
⁶³Cu stands out as the ubiquitous form of copper in our daily lives. It’s the isotope found in copper wires, plumbing, and various alloys. Its abundance and versatility make it the go-to choice for industrial and commercial applications.
Unveiling Copper’s Atomic Tapestry
The isotopic diversity of copper provides a glimpse into its atomic structure. By studying copper’s isotopes, scientists can unravel the intricate interplay of protons, neutrons, and electrons within its nucleus, paving the way for a deeper understanding of its chemical and physical properties.
Electron Count: A Balancing Act in Copper’s Atomic Symphony
In the realm of elements, each atom holds a unique blueprint that defines its identity and properties. This blueprint, known as the atomic structure, reveals the intricacies of an atom’s internal workings, including the delicate balance between protons and electrons.
Copper, an essential transition metal, exhibits a remarkable harmony within its atomic structure. As an element, it proudly possesses an atomic number of 29, signifying the presence of 29 protons within its nucleus. These protons, carrying positive charges, are the cornerstone of the atom’s identity, giving it its unique position in the periodic table.
Counterbalancing the positively charged protons are the negatively charged electrons that orbit the nucleus. In a neutral atom, the number of electrons must precisely match the number of protons, creating a delicate equilibrium. Like a celestial dance, electrons gracefully move around the nucleus, occupying specific energy levels known as orbitals.
In the case of copper, its 29 electrons mirror the 29 protons found in its nucleus. This harmonious dance ensures the atom’s overall neutrality, preventing any electrical imbalance. The electrons are distributed in a specific configuration, providing a glimpse into copper’s unique chemical properties.
Delving into Copper’s Electron Configuration: A Journey through Quantum Orbits
Copper, a versatile metal widely employed in countless applications, boasts a unique atomic structure that underpins its remarkable properties. To fully grasp its chemical nature, we must delve into the realm of electron configuration, an intricate arrangement of electrons within the atom.
Electron Configuration: A Blueprint of Electron Distribution
Imagine the atom as a miniature solar system, with the nucleus, housing protons and neutrons, at its core. Orbiting the nucleus are electrons, negatively charged particles that dance in a symphony of energy levels. The arrangement of these electrons, known as electron configuration, provides a precise map of their whereabouts within the atom.
Copper’s Electron Configuration: [Ar] 3d¹⁰ 4s¹
Copper, with an atomic number of 29, possesses 29 electrons. Its electron configuration is elegantly expressed as [Ar] 3d¹⁰ 4s¹. Let’s decode this enigmatic notation.
The Core: A Stable Foundation
The [Ar] portion indicates that the electrons occupying the inner energy levels are arranged in the same manner as those of the element argon. These core electrons, tightly bound to the nucleus, play a passive role in chemical reactions.
The 3d Orbitals: A Half-Filled Symphony
The 3d¹⁰ notation reveals that 10 electrons reside in the 3d orbitals, a set of five orbitals with similar energy levels. These orbitals form a remarkable configuration, with each orbital housing a pair of electrons.
The 4s Orbital: A Lone Wanderer
Finally, the 4s¹ indicates the presence of a solitary electron in the 4s orbital, an orbital with a higher energy level than the 3d orbitals. This lone electron plays a crucial role in determining copper’s chemical reactivity.
Unveiling the Significance
Understanding copper’s electron configuration is essential for comprehending its chemical behavior. The 10 d-electrons make copper a transition metal, a class of elements known for their ability to form complex compounds and exhibit variable oxidation states. Meanwhile, the lone 4s electron allows copper to form stable bonds with other atoms, contributing to its adaptability in various applications.