Unveiling the Proton Count in Copper: Copper, an essential transition metal, possesses a distinct atomic number of 29. The atomic number signifies the number of protons within an element’s nucleus and plays a crucial role in defining its identity and properties. Each copper atom boasts 29 protons, shaping its chemical behavior and influencing its practical applications, such as in electrical wiring or biological processes. Understanding the proton count in copper provides invaluable insights into its characteristics and enables us to harness its potential effectively.
- Begin with a captivating hook that highlights the importance of understanding the number of protons in copper.
- State the main focus of the blog post: “Exploring the Proton Count in Copper.”
Exploring the Proton Count in Copper: Unlocking the Secrets of the Red Metal
In the realm of chemistry, understanding the number of protons within an element’s atomic structure is crucial for deciphering its properties and behavior. Protons, the positively charged particles residing in the nucleus, play a pivotal role in defining an element’s identity. Let’s delve into the intriguing case of copper, a versatile metal whose atomic number holds the key to its fascinating characteristics.
The Atomic Number: A Defining Trait
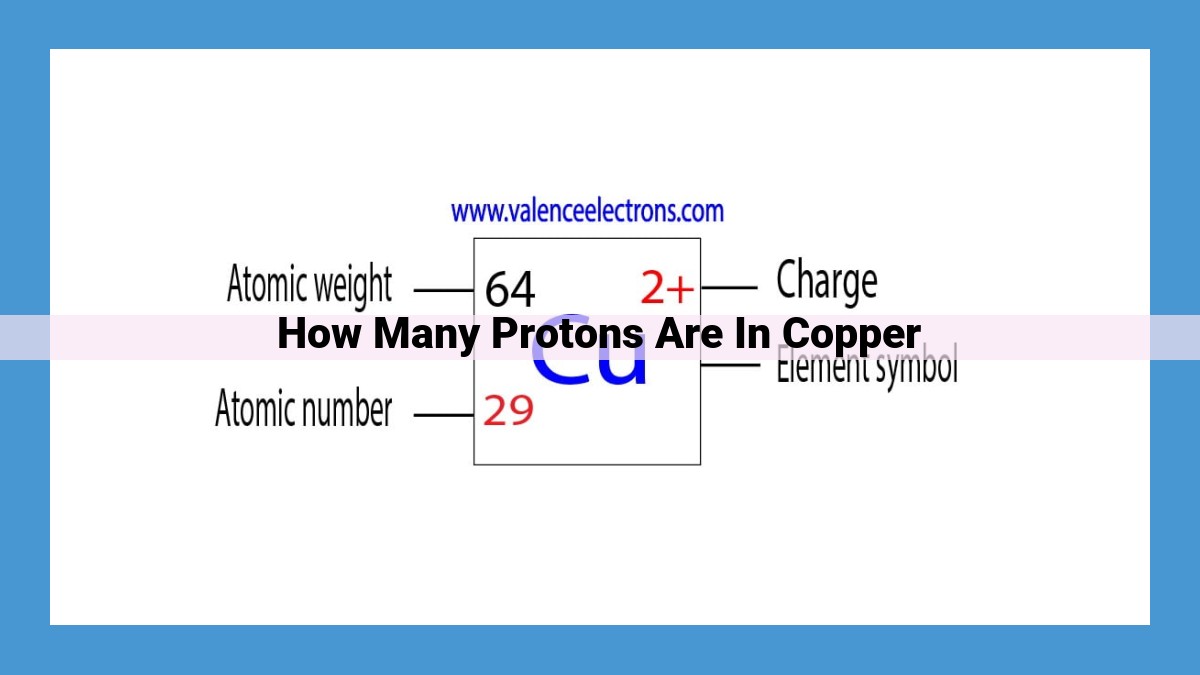
Every element in the periodic table is distinguished by its unique atomic number, which corresponds to the number of protons found in the nucleus of each atom. This number serves as a fingerprint, providing insight into an element’s chemical essence. For copper, this atomic fingerprint reads “29,” indicating that every single copper atom contains precisely 29 protons.
Copper’s Proton Count: A Gateway to Understanding
Copper, a transition metal, graces us with its reddish-orange hue and remarkable malleability. Its atomic number of 29 not only establishes its identity but also influences its electron configuration and reactivity. The arrangement of electrons around the nucleus, influenced by the number of protons, determines an element’s chemical properties.
Understanding the Essence of Atomic Number: A Key to Element Identity
In the vast tapestry of the universe, elements play a pivotal role as the building blocks of all matter. Each element possesses a unique identity defined by its atomic number, a fundamental concept that unveils the secrets of its composition.
Atomic Number: The Counting of Protons
Imagine an atom as a miniature solar system, with a nucleus at its core resembling a tiny sun. Within this nucleus, a fundamental particle known as protons resides. The number of protons present in the nucleus is what determines an element’s atomic number.
Significance of Atomic Number
This simple count of protons holds immense significance in determining the element’s identity. The atomic number dictates an element’s chemical properties, its position on the periodic table, and ultimately, its reactivity with other elements.
Each element possesses a distinct atomic number. For instance, hydrogen has an atomic number of 1, indicating that every hydrogen atom contains one proton in its nucleus. Similarly, oxygen boasts an atomic number of 8, revealing that each oxygen atom harbors eight protons in its core.
By understanding the atomic number of an element, scientists can uncover a wealth of information about its behavior and interactions. It serves as a cornerstone for comprehending the chemical reactions that shape our world and the properties of the materials that surround us.
Copper’s Atomic Number: Unveiling the Essence of the Red Metal
In the vast tapestry of elements, copper stands out as a transition metal with remarkable properties. Its unique characteristics, including its reddish hue and excellent electrical conductivity, have made it indispensable in various applications throughout history. At the heart of copper’s identity lies its atomic number, a fundamental property that defines its very essence.
Atomic number refers to the number of protons present in the nucleus of an atom. Protons, along with neutrons and electrons, constitute the building blocks of all matter. In the case of copper, its atomic number is 29. This means that each copper atom contains 29 protons in its nucleus.
Copper’s atomic number plays a crucial role in determining its electron configuration. Electrons, negatively charged particles that orbit the nucleus, are arranged in specific energy levels around the atom. The number of electrons in each energy level is determined by the atomic number. In copper, the 29 protons in the nucleus attract 29 electrons to maintain a neutral charge.
The electron configuration of copper directly influences its chemical properties and reactivity. The outermost electrons, known as valence electrons, are involved in chemical bonding and determine how copper interacts with other elements. Copper’s 11 valence electrons make it a relatively reactive metal, readily forming bonds with other elements.
Understanding the atomic number of copper is essential for comprehending its practical applications. In electrical wiring, copper’s high electrical conductivity makes it an ideal material for conducting electricity. Its ductility and malleability allow it to be easily drawn into wires and shaped into various components.
In biology, copper plays a vital role in numerous processes. It is a cofactor for several enzymes involved in oxygen transport and energy production. The correct number of protons in copper is crucial for these processes to function properly.
In conclusion, copper’s atomic number of 29 is the cornerstone of its unique properties. It determines the element’s electron configuration, chemical reactivity, and practical applications. Understanding this fundamental aspect of copper provides a deeper understanding of its behavior and significance in various fields.
Understanding the Intimate Relationship between Protons and Copper’s Nature
Copper, a captivating transition metal, holds a special allure in the realm of elements due to its unique atomic number of 29. Delving into this numerical essence is a gateway to unlocking the secrets of copper’s fascinating chemical properties.
At its heart, the atomic number reigns supreme as the defining characteristic of an element, dictating the fundamental building blocks of its identity. It represents the precise number of protons residing within the nucleus of each atom. In the case of copper, each atom proudly boasts 29 protons, a characteristic that sets it apart from all other elements.
This proton count exerts a profound influence on copper’s electron configuration, shaping its chemical behavior and reactivity. Remember, protons carry a positive charge, while electrons bear a corresponding negative charge. The dance between these charged particles is a delicate balancing act, determining the element’s ability to form bonds and interact with its surroundings.
With 29 protons in its nucleus, copper finds equilibrium with 29 electrons orbiting around it. Arranged in distinct energy levels, or shells, these electrons orchestrate copper’s chemical symphony. The outermost electrons, known as valence electrons, play a pivotal role in chemical reactions, determining how readily copper can share, donate, or accept electrons.
Understanding the intricate interplay between copper’s proton count and electron configuration is crucial for comprehending its diverse applications. For instance, in electrical wiring, copper’s unique ability to conduct electricity stems from the mobility of its valence electrons. Copper’s high thermal conductivity, a boon in cookware, also finds its roots in this electronic structure.
The atomic number of 29 not only defines copper’s chemical properties but also shapes its biological significance. As an essential trace element, copper plays a vital role in numerous physiological processes, including energy production, antioxidant defense, and nerve function. Its ability to interact with biological molecules, influenced by its proton count, underscores its importance in maintaining cellular health.
In essence, the proton count of 29 is the cornerstone of copper’s identity, shaping its physical, chemical, and biological attributes. By unraveling this intimate relationship, we gain a deeper appreciation for the remarkable properties that make copper an indispensable element in our technological and biological landscapes.
Practical Applications of Understanding the Proton Count in Copper
The knowledge of the number of protons in copper is not only confined to theoretical understanding but has far-reaching practical applications. Copper’s unique atomic properties make it an essential component in various fields.
One of the most prominent uses of copper is in electrical wiring. The exceptional conductivity of copper, attributed to its 29 protons, enables it to efficiently transfer electrical current. Copper’s remarkable ability to conduct electricity makes it the ideal choice for power transmission lines, electrical appliances, and electronic devices. Without a firm grasp of copper’s proton count, the development of such technologies would be severely hindered.
Copper also plays a vital role in biological processes. As an essential trace element, it contributes to the formation of hemoglobin, the oxygen-carrying protein in red blood cells. The specific number of protons in copper allows it to bind to oxygen molecules effectively and facilitate their transport throughout the body. Understanding the proton count in copper is thus crucial for comprehending its biological significance and treating copper-related health conditions.
In addition to electrical wiring and biological functions, copper finds applications in various industries. Its malleability, ductility, and corrosion resistance make it ideal for manufacturing pipes, sheets, and other construction materials. Furthermore, copper’s antimicrobial properties make it suitable for use in medical equipment and antimicrobial surfaces.
In summary, understanding the number of protons in copper is essential for harnessing its unique properties and leveraging them in a wide range of practical applications. From electrical wiring to biological processes and industrial uses, copper’s specific atomic number plays a pivotal role in shaping its characteristics and defining its contributions to various fields.