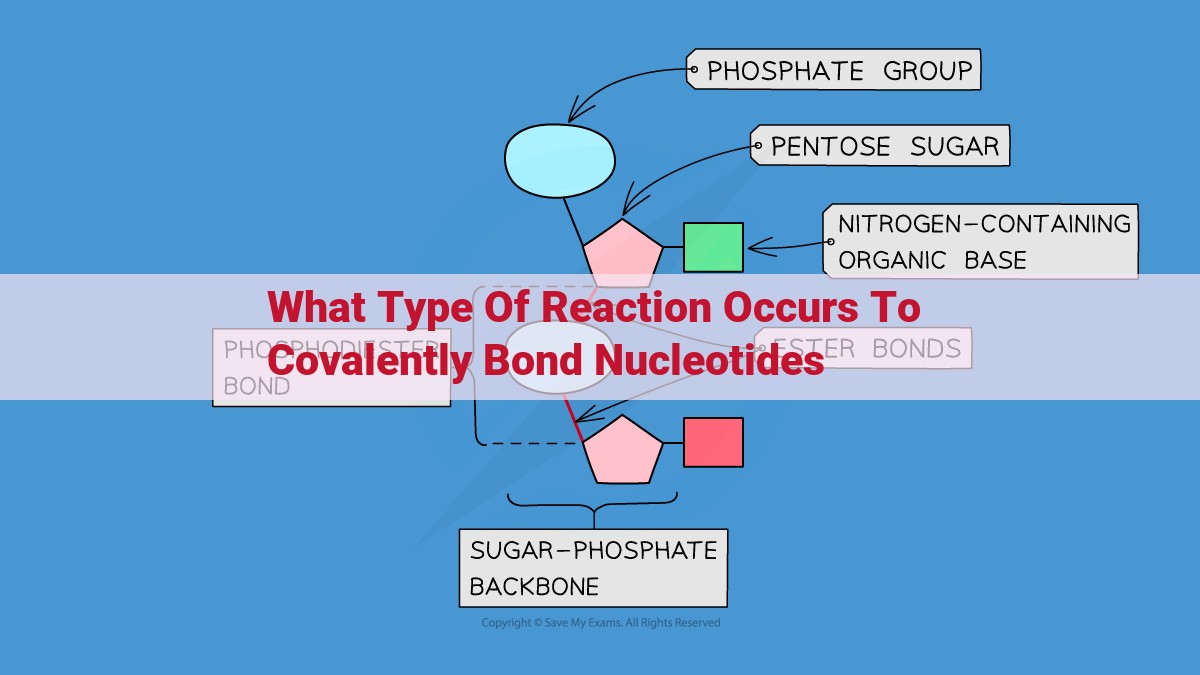
In dehydration synthesis, nucleotides are joined covalently through a phosphodiester bond. The hydroxyl group of one nucleotide acts as a nucleophile, attacking the electrophilic phosphate group of another nucleotide. This nucleophilic attack results in the formation of a phosphodiester bond, releasing a molecule of water as a byproduct. The hydroxyl group of the first nucleotide becomes the nucleophile and attacks the phosphate group on a second nucleotide, repeating the process. This reaction leads to the formation of a continuous chain of nucleotides, forming the backbone of a nucleic acid molecule.
Nucleotides: The Foundation of Life’s Blueprints
Let’s embark on a captivating journey into the molecular realm, where we unravel the secrets of nucleotides, the fundamental building blocks of life’s blueprints – nucleic acids. These intricate biomolecules form the backbone of our genetic code, carrying the blueprints for all living organisms.
Nucleotides consist of a sugar molecule, a phosphate group, and a nitrogenous base. These components are essential for the formation of DNA and RNA, the two main types of nucleic acids. DNA stores genetic information, while RNA plays a crucial role in protein synthesis.
As we delve deeper into the molecular world, we encounter dehydration synthesis, the ingenious process that covalently binds nucleotides together. This intricate reaction involves a nucleophilic attack, where the hydroxyl group of one nucleotide forms a bond with the phosphate group of another nucleotide.
The hydroxyl group acts as the nucleophile, an electron-rich atom or group of atoms that seeks positive charges. The phosphate group, on the other hand, serves as the electrophile, an electron-poor atom or group of atoms that attracts negative charges.
This nucleophilic attack triggers a cascade of events, culminating in the formation of a phosphodiester bond. This strong covalent bond links the nucleotides in a chain, forming the backbone of nucleic acids.
The hydroxyl group, with its inherent nucleophilic nature, plays a pivotal role in dehydration synthesis. Its ability to attack the phosphate group sets in motion the sequence that ultimately binds nucleotides together.
Phosphodiester bonds, the pivotal connections between nucleotides, are responsible for the stability and integrity of nucleic acid structures. They form the backbone that supports the nitrogenous bases, which carry the genetic code.
So, as we delve into the intricate world of nucleotides and dehydration synthesis, we uncover the fundamental building blocks of life. Nucleotides, with their unique structural components, combine through dehydration synthesis to form the very fabric of our genetic blueprint – the backbone of all living organisms.
Dehydration Synthesis: The Process of Covalently Bonding Nucleotides
- Describe dehydration synthesis as the type of reaction that bonds nucleotides together.
- Explain the steps involved in dehydration synthesis, including the nucleophilic attack and phosphodiester bond formation.
Dehydration Synthesis: The Covalent Dance of Nucleotides
In the realm of molecular biology, the formation of nucleic acids, the blueprints of life, is a captivating dance of nucleotides. These fundamental building blocks of DNA and RNA play a crucial role in transmitting genetic information across generations. To understand this intricate process, let’s delve into the mesmerizing tale of dehydration synthesis.
The Covalent tango of Nucleotides
Dehydration synthesis is the type of chemical reaction that covalently binds nucleotides together, forming the backbone of nucleic acids. This elegant dance begins with the encounter of two nucleotides, each composed of a nitrogenous base, a pentose sugar, and a phosphate group.
The Nucleophilic Attack: A Calculated Assault
The decisive moment in dehydration synthesis is the nucleophilic attack. A nucleophile is an electron-rich molecule, while an electrophile is an electron-deficient molecule. In our story, the hydroxyl group (OH) of one nucleotide, armed with its surplus of electrons, acts as the nucleophile. It lunges at the phosphate group (PO4) of another nucleotide, the unfortunate electrophile.
The Phosphodiester Bond: The Enduring Legacy
The nucleophilic attack triggers a cascade of events leading to the formation of a phosphodiester bond. This bond is the enduring link between the nucleotides, akin to a covalent handshake sealing their union. A water molecule is released as a byproduct of this chemical dance, hence the term “dehydration synthesis.”
The Hydroxyl Group: A Silent Accomplice
The hydroxyl group of the attacking nucleotide, initially a passive bystander, plays a crucial role as the nucleophile. Its eagerness to donate electrons initiates the nucleophilic attack, setting the stage for the phosphodiester bond formation.
The Phosphate Group: A Reluctant Participant
As the electrophile, the phosphate group of the target nucleotide initially resists the advances of the hydroxyl group. However, its electronegative core, starved for electrons, eventually acquiesces to the nucleophilic attack, resulting in the formation of a stable covalent bond.
Through this intricate dance of dehydration synthesis, nucleotides join forces to create nucleic acids, molecules that hold within them the secrets and blueprints of life. These covalent bonds, forged in the molecular crucible of cells, ensure the faithful transmission of genetic information across generations.
Nucleophilic Attack: The Pivotal Step in Dehydration Synthesis
In the realm of molecular biology, genetic information hinges upon the intricate dance of nucleic acids. These colossal molecules, composed of nucleotides, serve as blueprints for life, orchestrating the synthesis of proteins that govern our very existence. Nucleotides, the fundamental building blocks of nucleic acids, are linked together through a process known as dehydration synthesis, a chemical reaction that forges the backbone of these genetic giants.
At the heart of dehydration synthesis lies a pivotal step, a nucleophilic attack, a molecular handshake that unites nucleotides into a cohesive strand. Let’s delve into the captivating world of nucleophiles and electrophiles, unraveling their roles in this crucial biochemical reaction.
Unveiling Nucleophiles and Electrophiles: A Molecular Tango
Imagine a tale of two molecular entities: nucleophiles and electrophiles. Nucleophiles, the eager dancers, possess an innate craving for electrons, while electrophiles, the obliging partners, readily offer their electrons to fulfill this desire. In our story, the hydroxyl group of one nucleotide, armed with its spare electrons, serves as the nucleophile. On the other side of the dance floor stands the phosphate group of another nucleotide, an electrophile eager to share its electrons.
The Nucleophilic Attack: A Molecular Embrace
As the dance intensifies, the hydroxyl group, driven by its insatiable hunger for electrons, launches an attack on the unsuspecting phosphate group. With precision and grace, the hydroxyl group’s electrons lunge towards the phosphate group’s positively charged phosphorus atom. In this electrifying embrace, a phosphodiester bond is formed, linking the two nucleotides together.
The phosphodiester bond, a testament to the nucleophilic attack, becomes the backbone of the ever-growing nucleic acid molecule. This molecular bond serves as a sturdy bridge, connecting nucleotides in a linear chain, carrying the genetic code that shapes life as we know it.
Phosphodiester Bond Formation: The Result of Dehydration Synthesis
In the realm of molecular biology, the formation of phosphodiester bonds plays a crucial role in creating the building blocks of life. Dehydration synthesis, a fundamental chemical reaction, orchestrates this process, meticulously linking nucleotides together to form the very strands that carry our genetic code.
During dehydration synthesis, a nucleophile, eager to donate its electrons, attacks an electrophile, hungry to receive them. In this dance of molecular chemistry, the hydroxyl group of one nucleotide, acting as the nucleophile, makes a daring advance toward the phosphate group of another nucleotide, the designated electrophile.
This encounter gives rise to a new covalent bond, the phosphodiester bond, which connects the sugar group of one nucleotide to the phosphate group of the next, forming a sturdy bridge. This covalent bond is the backbone of nucleic acids, DNA and RNA, the molecules that hold the blueprint of our existence.
The end product of dehydration synthesis is a polymer, a long chain of nucleotides held together by a series of phosphodiester bonds. These polymers, with their immense length and complexity, carry the genetic information that governs every aspect of our being, from our physical appearance to our susceptibility to disease.
The Hydroxyl Group: The Nucleophile in Dehydration Synthesis
In the realm of molecular biology, the dance of nucleotides takes center stage. These building blocks of nucleic acids are joined together through a process called dehydration synthesis, a symphony of chemical reactions that create the intricate tapestry of DNA and RNA. At the heart of this dance is a pivotal player: the hydroxyl group, a nucleophile that initiates a crucial step in dehydration synthesis.
Defining the Hydroxyl Group
The hydroxyl group is an oxygen atom bonded to a hydrogen atom. It resides within the nucleotides, dangling off the five-carbon sugar backbone. This tiny yet potent group possesses a negative charge and a strong affinity for protons.
The Nucleophilic Attack: A Chemical Pas de Deux
In dehydration synthesis, the hydroxyl group serves as the nucleophile. It eagerly seeks a partner, an electrophile, to engage in a chemical dance. The electrophile in this case is the phosphate group of another nucleotide.
The hydroxyl group, with its negative charge, lunges toward the positive phosphorus atom of the phosphate group. This nucleophilic attack is the catalyst for a remarkable transformation.
The Birth of the Phosphodiester Bond
The nucleophilic attack triggers a cascade of events. The oxygen atom of the hydroxyl group grabs onto the phosphorus atom, breaking the bond between phosphorus and oxygen. This action releases a water molecule as a byproduct.
In its place, a robust phosphodiester bond emerges, a covalent bond that unites the two nucleotides. This bond forms the backbone of nucleic acids, providing the structural integrity and information-carrying capacity essential for life.
The Hydroxyl Group’s Essential Role
Without the hydroxyl group, the dance of dehydration synthesis would falter. Its nucleophilic nature sets the stage for the nucleophilic attack, the pivotal step that forges the phosphodiester bonds. These bonds, in turn, construct the intricate architecture of nucleic acids, the blueprints for life.
Phosphate Group: The Electrophile in Dehydration Synthesis
In the intricate dance of nucleotide bonding, there’s a player known as the phosphate group, the electrophile that sets the stage for the covalent bonding of nucleotides.
What is a Phosphate Group?
A phosphate group, symbolized as PO4 3–, is a negatively charged ion composed of phosphorus and oxygen atoms. In the context of nucleic acids, the phosphate group forms the backbone of the nucleotide chain.
Role in Dehydration Synthesis
During dehydration synthesis, the phosphate group plays a crucial role as the electrophile, meaning it attracts an electron-rich species, known as a nucleophile, to form a covalent bond.
This happens through a nucleophilic attack, where the hydroxyl group (_OH–) of one nucleotide acts as the nucleophile and attacks the phosphate group of another nucleotide. The result is the formation of a phosphodiester bond, the key structural element of nucleic acid polymers like DNA and RNA.