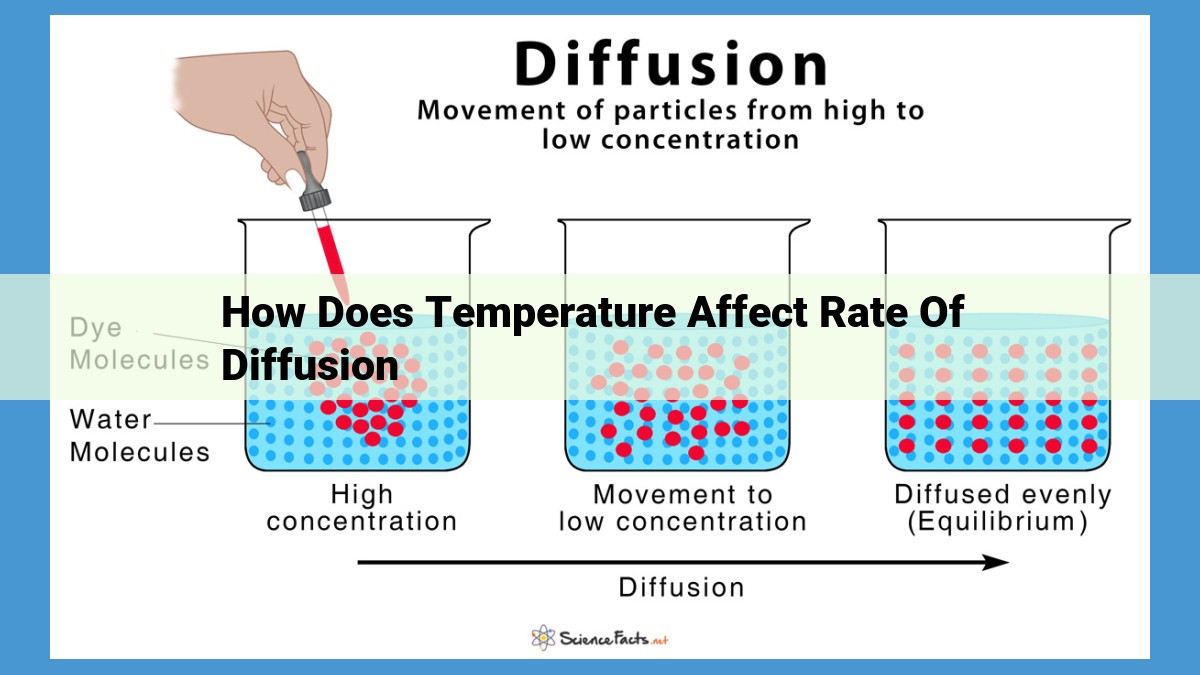
Diffusion, the movement of particles from high concentration areas to low, is crucial in biological and chemical systems. Temperature significantly impacts diffusion by influencing the kinetic energy of particles. Higher temperatures increase kinetic energy, enabling particles to overcome the activation energy barrier, leading to a faster diffusion rate. This relationship is exponential due to the lowered activation energy at elevated temperatures. Understanding this relationship is essential in fields such as environmental science, where temperature manipulation controls diffusion rates for pollutant remediation, and in medicine, where drug delivery is optimized by varying temperatures to enhance drug diffusion into target cells.
The Intriguing Dance of Diffusion: Temperature’s Orchestration
Diffusion, the mesmerizing dance of molecules relentlessly moving from higher to lower concentrations, plays a pivotal role in the symphony of life. From the absorption of nutrients in our cells to the release of waste products, diffusion is the driving force behind countless biological processes. In the realm of chemistry, diffusion governs the mixing of reactants and the formation of new compounds.
A Burning Question
A compelling question lingers: how does temperature, the fiery conductor of molecular motion, influence the graceful waltz of diffusion? This blog post embarks on an illuminating journey to unravel this intriguing relationship, exploring the profound impact of temperature on the rate of diffusion.
Key Concepts: The Ingredients for Understanding
To comprehend the dance between temperature and diffusion, six key concepts will illuminate our path:
- Rate of diffusion: The velocity of molecules’ movement from high to low concentrations.
- Temperature: The measure of molecular kinetic energy, the frantic vibrations and collisions that drive diffusion.
- Kinetic energy: The energy of motion, propelling molecules through space.
- Activation energy: The energy barrier that molecules must overcome to initiate diffusion.
- Diffusion coefficient: A measure of the ease with which molecules diffuse through a medium.
- Temperature dependence of diffusion: The relationship between temperature and the rate of diffusion, revealing the profound influence of heat on molecular mobility.
Six Key Concepts: Dissecting the Influence of Temperature on Diffusion
When molecules embark on their journey of diffusion, temperature becomes their maestro, dictating the pace at which they traverse space. To unravel the intricate relationship between these two entities, we introduce six key concepts that will illuminate our path.
1. Rate of Diffusion:
Imagine a crowd of people shuffling through a doorway; the rate of diffusion refers to how quickly they cross the threshold. In our molecular world, it measures the speed at which particles spread from an area of high concentration to low concentration.
2. Temperature:
Like a conductor’s baton, temperature orchestrates the dance of molecules. It is a measure of the average kinetic energy of the particles within a system.
3. Kinetic Energy:
Kinetic energy is the energy an individual molecule possesses due to its motion. Higher temperatures translate to higher kinetic energy, leading to more energetic and spritely particles.
4. Activation Energy:
Think of activation energy as the initial hurdle molecules must overcome to start diffusing. It represents the minimum energy required for molecules to break free from their current locations and embark on their journey.
5. Diffusion Coefficient:
The diffusion coefficient quantifies the ease with which molecules can diffuse through a given medium. It is affected by factors such as molecular size, temperature, and the nature of the medium itself.
6. Temperature Dependence of Diffusion:
This concept highlights the profound impact temperature has on diffusion rates. As temperature increases, kinetic energy soars, activation energy dips, and the diffusion coefficient amplifies, resulting in a marked increase in the rate of diffusion.
Influence of Temperature on Rate of Diffusion:
- Discuss how temperature affects the kinetic energy of particles and the subsequent effect on the rate of diffusion.
- Provide examples to illustrate the relationship between increasing temperature and increased rate of diffusion.
Influence of Temperature on Rate of Diffusion
How Temperature Propels the Dance of Molecules
In the realm of science, diffusion reigns supreme, orchestrating the intricate movement of molecules from high to low concentrations. Like ballerinas gliding across a stage, molecules follow a graceful dance determined by the temperature of their surroundings. As the temperature rises, the tempo of this dance quickens, leading to a significant increase in the rate of diffusion.
Temperature: The Maestro of Kinetic Energy
Temperature exerts its influence by manipulating the kinetic energy of molecules. Kinetic energy, the energy of motion, determines the speed and agility of these tiny dancers. As temperature increases, so does their kinetic energy, propelling them with greater velocity. This surge in energy enables molecules to overcome barriers and traverse distances more swiftly, ultimately accelerating the diffusion process.
Examples: Temperature-Dependent Diffusion in Action
Consider the diffusion of perfume molecules in a room. On a sweltering summer day, the perfume’s fragrant molecules dance with vigor, permeating the air with an intense aroma. Conversely, on a chilly winter night, their movement is sluggish, resulting in a faint, ethereal scent.
Another example is the diffusion of oxygen in a cell. As the body’s temperature rises during exercise, so does the rate of oxygen diffusion into cells, meeting the increased metabolic demands. This surge in oxygen supply fuels the muscles, enabling them to perform at their peak.
The relationship between temperature and rate of diffusion is a fundamental principle that governs countless biological and chemical processes. From the diffusion of nutrients into cells to the spread of pollutants in the environment, temperature plays a crucial role in shaping the pace of these molecular movements. Understanding this relationship is essential for scientists and researchers seeking to manipulate and control diffusion rates in various fields, such as medicine, environmental science, and chemical engineering.
Activation Energy and Temperature’s Impact on Diffusion
Diffusion, the movement of molecules from an area of high concentration to an area of low concentration, plays a crucial role in biological and chemical processes. Temperature, a measure of the average kinetic energy of particles, significantly influences the rate of diffusion.
Activation energy is the minimum amount of energy required for a reaction to occur. In the context of diffusion, it represents the energy barrier that molecules must overcome to move from one position to another.
Temperature exerts a profound effect on activation energy:
-
Higher temperature: Increases the kinetic energy of molecules, making it easier for them to overcome the activation energy barrier. As a result, the rate of diffusion increases exponentially.
-
Lower temperature: Decreases the kinetic energy of molecules, making it more challenging for them to overcome the activation energy barrier. Consequently, the rate of diffusion decreases.
This relationship between temperature and activation energy is crucial for understanding and controlling diffusion in various fields:
-
Environmental science: Manipulation of temperature can be used to enhance or inhibit diffusion processes that affect pollutant remediation or nutrient cycling.
-
Medicine: Temperature control is essential in drug delivery and biomedical imaging, where diffusion rates influence drug efficacy and diagnostic accuracy.
-
Chemical engineering: Temperature optimization is used in separation processes, crystallization, and catalysis, where diffusion plays a critical role in reaction rates and product quality.
By understanding the temperature dependence of diffusion and its implications on activation energy, scientists and engineers can harness this knowledge to enhance diffusion-based processes and advancements.
Significance and Applications of Temperature-Dependent Diffusion
Environmental Science:
Understanding the impact of temperature on diffusion is crucial in environmental science. Fluctuating temperatures can alter the diffusion rates of pollutants in air and water. For instance, increased temperatures accelerate the diffusion of greenhouse gases, exacerbating climate change. By manipulating temperatures, scientists can control the movement of pollutants, mitigating their environmental impact.
Medicine:
In medicine, temperature manipulation is pivotal in drug delivery. Controlling the temperature of injectable solutions can influence the diffusion of drugs into specific tissues or organs. This targeted delivery ensures effective treatment while minimizing side effects. Additionally, temperature-controlled diffusion plays a role in hyperthermia and hypothermia treatments, where temperatures are manipulated to induce specific therapeutic responses.
Chemical Engineering:
In chemical engineering, temperature is a critical variable in industrial processes. Temperature control influences the diffusion rates of reactants and products, affecting chemical reaction yields and efficiency. By optimizing temperatures, engineers can maximize reaction rates, reduce production time, and enhance product quality. For example, in membrane separation, temperature manipulation is used to control the diffusion of gases and liquids, enabling selective separation processes.
The relationship between temperature and diffusion is a fundamental principle with far-reaching applications. By understanding this relationship, scientists and engineers can manipulate diffusion rates to address a wide range of challenges in fields such as environmental science, medicine, and chemical engineering. This knowledge empowers us to optimize processes, control pollutant movement, deliver drugs effectively, and advance various technologies.