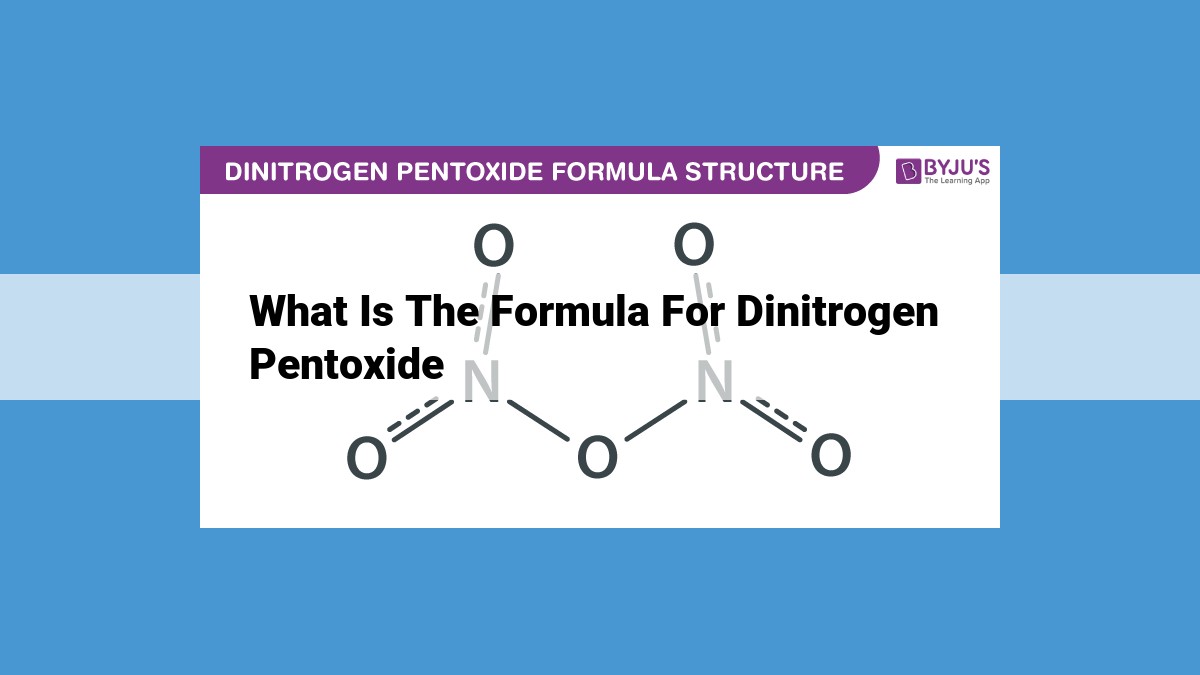
Dinitrogen pentoxide, represented by the chemical formula N₂O₅, comprises two nitrogen atoms and five oxygen atoms per molecule. Its molecular weight is 108.01 g/mol, with each nitrogen atom contributing 14.01 g/mol and each oxygen atom contributing 16.00 g/mol. According to IUPAC nomenclature, it is named dinitrogen pentoxide, reflecting its two nitrogen atoms and five oxygen atoms. Its structural formula depicts the bonding arrangement: O=N-O-N=O, showcasing alternating double bonds between nitrogen and oxygen atoms.
Unveiling the Chemical Identity of Dinitrogen Pentoxide: A Journey into Its Molecular Makeup
Embark on an intriguing exploration into the chemical realm, where we decipher the enigmatic formula of dinitrogen pentoxide. This captivating compound, denoted by the chemical formula N₂O₅, holds secrets about its molecular composition and structure that await our discovery.
N₂O₅, a tantalizing blend of elements, unveils its atomic constituents: two nitrogen atoms and five oxygen atoms. These atoms dance together, forming a molecular entity that possesses unique properties and plays a significant role in various chemical processes.
Unveiling the Molecular Weight and Composition of Dinitrogen Pentoxide
The Molecular Weight Conundrum
Imagine you’re holding a tiny molecular scale, eagerly awaiting the weight of dinitrogen pentoxide, a molecule composed of nitrogen and oxygen atoms. To determine this weight, we must first understand its molecular formula: N₂O₅. This formula tells us that each molecule contains 2 nitrogen atoms (N) and 5 oxygen atoms (O).
Breaking Down the Molecular Weight
The molecular weight of a molecule is like a sum of weights of all its constituent atoms. Each element has its own unique atomic weight, expressed in atomic mass units (amu). Nitrogen has an atomic weight of 14 amu, while oxygen weighs in at 16 amu.
So, for dinitrogen pentoxide, we can calculate its molecular weight as:
Molecular weight = (Number of N atoms) x (Atomic weight of N) + (Number of O atoms) x (Atomic weight of O)
Molecular weight = (2 x 14 amu) + (5 x 16 amu)
Molecular weight = **108 amu**
Therefore, the molecular weight of dinitrogen pentoxide is 108 amu.
Constituent Atoms and Their Weights
To summarize, the molecular weight of 108 amu for dinitrogen pentoxide corresponds to the following constituent atoms:
- 2 Nitrogen atoms (N), each weighing 14 amu, contributing 28 amu
- 5 Oxygen atoms (O), each weighing 16 amu, contributing 80 amu
This breakdown helps us understand the molecular composition and the relative contributions of different atoms to the overall weight of the molecule.
IUPAC Name: Unveiling the Chemical Identity of Dinitrogen Pentoxide
The International Union of Pure and Applied Chemistry (IUPAC) has established a systematic naming system for chemical compounds to ensure clarity and consistency in their identification. This system plays a crucial role in scientific communication and helps researchers effortlessly understand the structure and composition of molecules.
For dinitrogen pentoxide, the IUPAC name reflects its molecular composition. It begins with the prefix “di,” indicating the presence of two nitrogen atoms. The root “nitrogen” specifies the element, and the suffix “pentoxide” signifies the presence of five oxygen atoms.
The IUPAC name for dinitrogen pentoxide is nitrogen pentoxide. This name precisely conveys that the molecule contains two nitrogen atoms and five oxygen atoms. The prefixes and suffixes used in the name provide valuable information about the number and types of atoms present, making it an essential tool for scientists to identify and describe chemical compounds.
Deciphering the Structural Formula of Dinitrogen Pentoxide
Revealing the Molecular Architecture
The structural formula of a molecule provides a visual representation of its atomic connectivity. In the case of dinitrogen pentoxide, its structural formula uncovers the intricate arrangement of its atoms, unveiling the molecular architecture that defines its properties.
Delving into the Structural Formula
The structural formula of dinitrogen pentoxide, N₂O₅, resembles a molecular blueprint. It depicts two nitrogen atoms bound together by a triple bond, forming the central backbone of the molecule. Extending from each nitrogen atom are two oxygen atoms, each connected by double bonds. These double bonds give rise to the “pentoxide” in the molecule’s name, indicating the presence of five oxygen atoms.
Unveiling the Types of Bonds
The structural formula not only reveals the connectivity of atoms but also hints at the types of bonds present within the molecule. The triple bond between the nitrogen atoms is one of the strongest covalent bonds in chemistry, contributing significantly to the stability of the molecule. The double bonds between nitrogen and oxygen, on the other hand, are weaker than triple bonds but still confer a degree of rigidity to the molecular structure.
Arranging the Atoms with Precision
The structural formula of dinitrogen pentoxide showcases the precise arrangement of its atoms in space. The two nitrogen atoms lie along a linear axis, with the oxygen atoms positioned at the corners of a square plane. This arrangement is dictated by the hybridization of the nitrogen atoms, which adopt a trigonal planar geometry, allowing for efficient bonding with the surrounding oxygen atoms.
Other Chemical Identifiers for Dinitrogen Pentoxide: A Chemical Name Detective’s Guide
Beyond its systematic name and structural formula, dinitrogen pentoxide is also known by a plethora of chemical identifiers. These unique codes and notations play a pivotal role in the scientific community, providing a standardized way to reference and track chemical substances. Just like secret agent code names, these identifiers help scientists identify and communicate about this enigmatic compound with precision.
One such identifier is the CAS number. Imagine a chemical fingerprint, the CAS number is a unique numerical code assigned to every known chemical substance. For dinitrogen pentoxide, its CAS number is 10102-03-1. This code is like a secret agent’s dossier, providing a quick and accurate way to pull up all the vital information about this compound.
Another important identifier is the PubChem CID. Short for “Public Chemistry Identifier,” PubChem CID is another unique number assigned to chemical substances. It’s like a passport number for chemicals, allowing scientists to access a wealth of data and resources associated with dinitrogen pentoxide on the PubChem database.
For those who prefer a more compact code, SMILES (Simplified Molecular Input Line Entry System) comes into play. SMILES is a string of characters that represents the molecular structure of dinitrogen pentoxide. Think of it as a cryptic chemical shorthand that allows scientists to quickly sketch the molecule’s blueprint.
InChI (International Chemical Identifier) and InChIKey are two other identifiers that provide a more detailed description of dinitrogen pentoxide’s molecular structure. InChI is like a chemical recipe, capturing all the atoms, bonds, and even the 3D arrangement of the molecule. InChIKey, on the other hand, is a unique hashed version of InChI that’s shorter and easier to handle. It’s like a chemical shorthand for InChI, allowing scientists to quickly search and compare molecular structures.
These chemical identifiers are not mere academic curiosities. They are essential tools for scientists, researchers, and industry professionals involved in the discovery, development, and use of chemical substances. They help streamline communication, ensure accuracy, and facilitate the exchange of scientific knowledge. So, the next time you encounter dinitrogen pentoxide or any other chemical compound, remember the power of chemical identifiers. They are the secret code names that help scientists navigate the vast world of chemistry.