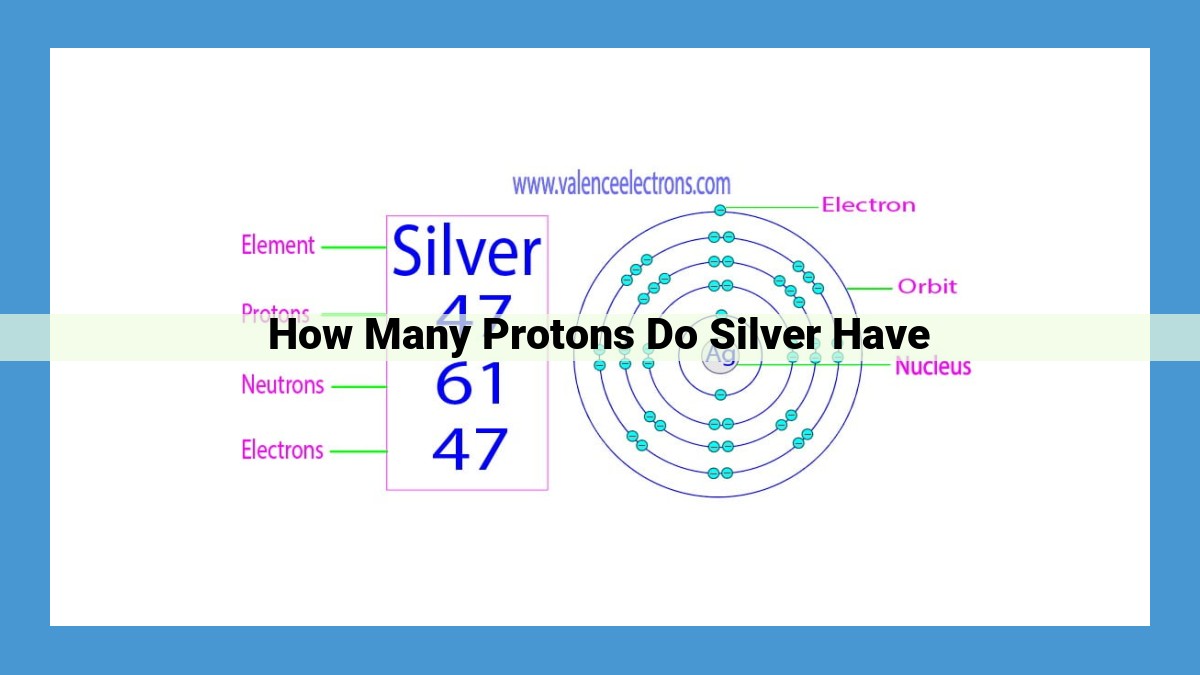
The number of protons in an atom’s nucleus defines its identity, known as the atomic number. Each atom of an element has the same number of protons, which uniquely identifies that element. Silver’s atomic number, represented by its symbol Ag, is 47, indicating that each silver atom contains 47 protons.
Understanding the Atomic Number: The Key to an Atom’s Identity
In the realm of science, the smallest building blocks of matter are atoms. Each atom is composed of a nucleus, which contains positively charged protons and neutral neutrons, and electrons that orbit the nucleus. Among these particles, the protons play a pivotal role in defining an atom’s identity, and their number is known as the atomic number.
The atomic number is like the fingerprint of an atom. It represents the number of protons found within the nucleus. Each element, a pure substance composed of atoms with the same atomic number, has its own unique atomic number. For instance, hydrogen has an atomic number of 1, which means every hydrogen atom contains one proton in its nucleus. Helium, on the other hand, has an atomic number of 2, indicating that each helium atom has two protons.
Protons are the heavyweights of the atomic nucleus. Their positive charge is responsible for balancing the negative charge of the electrons orbiting the nucleus. The number of protons in an atom determines its atomic number and, consequently, its identity as an element. Without protons, an atom would cease to exist, and the element itself would lose its distinct characteristics.
The understanding of the atomic number is crucial for comprehending the fundamental nature of matter. It provides the basis for the organization of elements into the periodic table, where elements are arranged in ascending order of their atomic numbers. This arrangement highlights the similarities and differences between elements, allowing scientists to predict their chemical behavior and properties.
The Number of Protons: Defining an Element’s Identity
In the realm of atoms, protons play a pivotal role. These minuscule particles, nestled within an atom’s nucleus, hold the key to identifying and understanding various elements. The number of protons present in an atom’s nucleus is known as its atomic number.
Imagine atoms as tiny building blocks, each with a unique identity determined by its atomic number. This number serves as an immutable fingerprint that defines an element’s chemical properties. For instance, all atoms with six protons are carbon atoms, and all atoms with one proton are hydrogen atoms.
The significance of the atomic number cannot be understated. It serves as a unique identifier for each element, distinguishing it from all others. This fundamental property lays the foundation for understanding the organization and behavior of elements within the periodic table and the wider world of chemistry.
Element
- Explain that an element is a pure substance with atoms sharing the same atomic number (and protons).
- Describe the use of chemical symbols to represent elements.
Defining Elements: The Building Blocks of Matter
In the vast tapestry of nature’s building blocks, elements stand as the fundamental constituents, each possessing a unique and defining atomic number. This number, which represents the number of protons within an atom’s nucleus, plays a pivotal role in shaping the identity and properties of the element.
Protons, with their positive charge, occupy the core of every atom. Their presence, in specific numbers, determines the element’s place within the periodic table, establishing its chemical behavior and defining the properties that distinguish it from others.
To simplify understanding, imagine a group of lockers in a school hallway. Each locker is assigned a unique number, akin to the atomic number of an element. Within each locker, one finds a certain number of items, which can be likened to the number of protons in an atom. Just as the items within a locker define its contents, the number of protons in an atom defines the element to which it belongs.
Elements: Defining Identity and Distinctive Traits
An element is a pure substance comprising atoms with identical atomic numbers, meaning they contain the same number of protons. This singular characteristic establishes the element’s identity and behavior, distinguishing it from other elements.
To illustrate this concept, consider silver, an element with an atomic number of 47. Every single atom of silver contains 47 protons, a defining feature that sets it apart from other elements and determines its unique properties.
Chemical Symbols: Shorthand for Elements’ Identity
For the sake of convenience, scientists have devised a system of chemical symbols, which represent elements using one or two letters. These symbols provide a shorthand way to refer to elements and facilitate communication among scientists.
For instance, the chemical symbol for silver is Ag, derived from its Latin name, argentum. This symbol not only identifies silver but also encodes its atomic number within it—the subscript “47” indicates the presence of 47 protons in the nucleus of each silver atom.
Silver (Ag)
- Introduce silver as an element with atomic number 47.
- Explain that each silver atom contains 47 protons.
Silver: A Precious Metal with a Rich History
In the realm of elements, silver stands as a treasure both ancient and alluring. With an atomic number of 47, it holds a unique position in the periodic table, offering a fascinating story to unravel.
At the heart of every silver atom lies a nucleus teeming with protons. These tiny particles, carrying a positive charge, define an atom’s identity. The number of protons in an atom’s nucleus is what sets one element apart from another. For silver, this number is 47, making it distinctly different from all other elements.
This number of protons, which is also known as the atomic number, is what gives an element its very essence. It determines the element’s chemical properties and its place in the periodic table. Silver, with its atomic number of 47, belongs to the group of transition metals.
An element, in its purest form, is defined as a substance composed of atoms that share the same atomic number. Each atom of silver, therefore, contains 47 protons, making it a unique element. To represent these elements conveniently, scientists use chemical symbols, which are one- or two-letter abbreviations. For silver, the symbol is Ag, derived from its Latin name, Argentum.
This symbol, Ag, carries with it a wealth of information. It represents not only the atomic number of 47 but also the element’s unique identity. Silver, with its lustrous sheen and malleable nature, has been used for centuries in jewelry, coinage, and countless other applications. Its atomic number remains the cornerstone of its properties, making it a precious metal with a story as rich and enduring as its own elemental form.
The Symbol of Silver: An Ancient Link to a Modern Element
In the vast expanse of the periodic table, each element holds a unique identity defined by its atomic number – the number of protons nestled within its nucleus. Silver, a precious metal known for its gleaming radiance and versatile applications, bears the atomic number 47. This number, etched into the core of every silver atom, is reflected in its chemical symbol: Ag.
The symbol Ag, a testament to silver’s rich history, finds its roots in the Latin word argentum, meaning “white” or “shining.” This Latin name, which aptly captures the element’s shimmering nature, was bestowed upon it by the Romans, who marveled at its beauty and utilized it for creating exquisite jewelry and currency.
Beyond its Latin origins, the symbol Ag serves as a concise representation of silver’s atomic nature. The atomic number, 47, embedded within the symbol, conveys the number of protons residing in the nucleus of each silver atom. This number, like a fingerprint, uniquely defines silver and distinguishes it from all other elements in the periodic table.
The atomic number plays a pivotal role in determining an element’s properties and reactivity. In the case of silver, its 47 protons account for its silvery-white appearance, high electrical and thermal conductivity, and resistance to corrosion. Silver’s unique properties have made it indispensable in various industries, from electronics and photography to jewelry and medicine.
Thus, the symbol Ag, derived from ancient Latin and encompassing the atomic number 47, encapsulates the essence of silver. It’s a symbol that reflects both the rich history and the intrinsic nature of this versatile element, a reminder of the fascinating stories that lie hidden within the realm of chemistry.