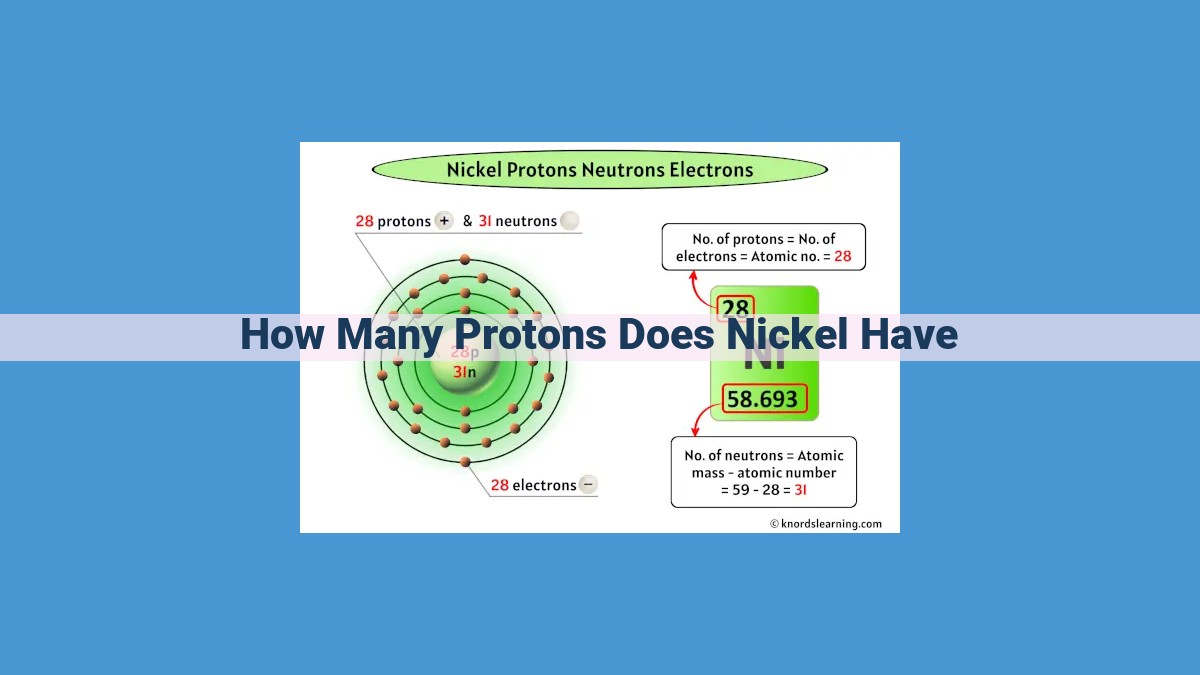
Nickel (Ni), with an atomic number of 28, is located in Period 4, Group 10 of the periodic table. Its atomic number, which defines the number of protons in an element’s nucleus, determines that Nickel has 28 protons. These positively charged particles, along with neutrons which have no electric charge, form the nucleus, while electrons orbit around it. Nickel’s electron configuration of [Ar] 3d8 4s2 reflects the distribution of its 28 electrons in specific energy levels and orbitals, which influences its chemical properties.
Nickel Unveiled: Exploring the Element’s Atomic Nature
In the vast tapestry of elements that make up our world, one captivating metal stands out – Nickel. With its silvery-white luster and remarkable properties, Nickel has played a pivotal role in shaping human civilization and industry.
Unveiling Nickel’s Atomic Identity
At the heart of every atom lies its atomic number, a fundamental property that defines an element’s unique identity. Nickel, a member of the transition metals, bears the atomic number 28. This number signifies that each Nickel atom possesses 28 protons, positively charged particles that reside within the atom’s nucleus. The atomic number not only distinguishes Nickel from all other elements but also determines its position within the periodic table, a roadmap of chemical elements.
Understanding Atomic Number and Protons: A Journey into the Heart of Nickel
In the vast tapestry of elements that compose our universe, Nickel stands out as a remarkably versatile metal with countless applications. To fully grasp its significance, we embark on a journey into the very core of this element, unraveling the secrets of its atomic number and the role of its protons.
Atomic Number: The Identity Key
Imagine the nucleus of an atom as a microscopic realm teeming with positively charged particles called protons and neutral particles known as neutrons. The atomic number of an element, represented by the symbol Z, is nothing more than the number of protons residing within its nucleus.
For Nickel, this number stands at 28, signifying its unique identity among the myriad elements. This information is crucial because the atomic number dictates the element’s position in the periodic table and its fundamental chemical properties.
The Nucleus: A Balancing Act
Within the nucleus, the protons play a pivotal role in determining an element’s character. They carry a positive electric charge, counterbalanced by the negative charges of the electrons orbiting the nucleus. This delicate balance maintains the atom’s stability and shapes its interactions with other elements.
In the case of Nickel, its 28 protons not only define its atomic number but also contribute to its overall stability. This stability allows Nickel to form strong bonds with other elements, making it a valuable component in alloys and various industrial applications.
Expanding Our Understanding
Our exploration into the atomic number and protons of Nickel offers a glimpse into the fundamental building blocks of matter. These tiny particles, hidden within the depths of atoms, hold the key to understanding the unique properties and behaviors of elements. As we delve deeper into the world of chemistry, the significance of atomic number and protons becomes increasingly apparent, guiding our understanding of the vast chemical universe around us.
Periodic Table
- Describe the periodic table as an arrangement of elements based on atomic number and properties.
- Indicate Nickel’s position in the table as Period 4, Group 10.
The Periodic Table: Nickel’s Home in the Symphony of Elements
In the realm of chemistry, the periodic table emerges as a majestic symphony of elements, each with its unique place and character. Among this extraordinary ensemble resides Nickel, an element with an atomic number of 28, occupying the hallowed halls of Period 4, Group 10.
The periodic table orchestrates its elements based on a meticulous system of order. Each element’s atomic number, the number of protons in its nucleus, determines its position within this grand tapestry. Nickel’s 28 protons, like so many musical notes, dictate its placement as the 28th element in the sequence.
Nickel’s residence in Period 4 reveals its energy level, the stage on which its electrons perform their dance. Within this layer, Nickel’s electrons harmonize, creating a unique set of chemical properties that distinguish it from its fellow elements.
Group 10, which Nickel calls home, unites elements with similar valence electrons, the outermost electrons that determine their chemical reactivity. These shared electrons create a common language among the elements within the group, influencing their ability to interact and form bonds with one another.
Protons, Neutrons, and Nucleons: The Heart of Nickel
In the bustling metropolis of the periodic table, we encounter a remarkable element known as nickel, bearing the symbol Ni and an atomic number of 28. This atomic number is no mere coincidence but a reflection of the protons residing within nickel’s nucleus.
Protons, with their unwavering positive charge, not only define nickel’s identity but also establish its position on the periodic table. These tiny particles reside at the very core of the atom, where they dance around, creating a vibrant center of positive energy.
But nickel’s nucleus is not a solitary realm for protons alone. Alongside them reside neutrons, neutral particles that, despite their lack of electric charge, play a pivotal role in the atom’s stability. Neutrons, like silent guardians, ensure that nickel’s nucleus remains intact, preventing it from succumbing to electrostatic repulsion.
The number of nucleons within nickel’s nucleus, comprising both protons and neutrons, determines its mass number. This mass number serves as the backbone of the atom, influencing its physical and chemical properties. Nucleons, acting in unison, provide the necessary mass for nickel to exist in its unique form.
Together, protons, neutrons, and nucleons form the very essence of nickel, dictating its atomic identity, position on the periodic table, and physical characteristics. These tiny particles, dwelling within the heart of nickel’s nucleus, are the architects of its existence, shaping the element that plays a vital role in our everyday lives.
Electron Configuration
- Explain electron configuration as the distribution of electrons in atomic orbitals.
- Describe Nickel’s electron configuration as [Ar] 3d8 4s2.
- Relate electron configuration to chemical properties.
Electron Configuration of Nickel: Unveiling the Inner Workings of an Element
Nickel, a versatile element with the atomic symbol Ni and atomic number 28, captivates scientists and engineers with its unique properties. At the heart of Nickel’s identity lies its electron configuration, a blueprint that reveals the arrangement of electrons within its atoms.
Electron configuration refers to the distribution of electrons in atomic orbitals, designated by the energy levels and shapes they occupy. Nickel’s electron configuration, expressed as [Ar] 3d8 4s2, provides valuable insights into its chemical behavior.
The [Ar] portion represents the electron configuration of Argon, the noble gas that precedes Nickel in the periodic table. This indicates that Nickel shares a similar electron arrangement with Argon in its core energy levels. The subsequent 3d8 notation signifies that Nickel has eight electrons in its d orbitals, which are a set of five orbitals with unique shapes and orientations. Finally, 4s2 denotes two electrons in the 4s orbital, the highest energy level in Nickel’s atom.
This specific electron configuration influences the chemical properties of Nickel. The presence of incompletely filled d orbitals allows Nickel to readily participate in chemical reactions, forming bonds with other atoms to achieve a stable electron configuration. Nickel’s ability to form various oxidation states, including +2 and +3, makes it a versatile component in alloys and catalysts.
By understanding Nickel’s electron configuration, scientists can predict its reactivity and tailor its properties for specific applications. From corrosion-resistant alloys to efficient catalysts, Nickel’s electron configuration plays a pivotal role in shaping its technological and industrial significance.