How Many Neutrons in Sodium: A Comprehensive Guide
Sodium, a crucial element in human biology and various industrial processes, possesses a specific neutron count that influences its atomic structure and properties. This blog post explores the concept of neutrons, explains how atomic number and mass number contribute to determining sodium’s neutron count, and delves into the variations among sodium isotopes. By understanding the intricacies of sodium’s neutron composition, we gain valuable insights into its behavior and applications in diverse fields.
How Many Neutrons Does Sodium Have? Understanding the Atomic Building Blocks
Sodium, an essential element in our daily lives, plays a crucial role in maintaining fluid balance, nerve function, and various physiological processes. To delve into the depths of sodium’s behavior and properties, it’s imperative to understand its atomic makeup, particularly the number of neutrons it harbors.
Atomic Identity and Neutrons
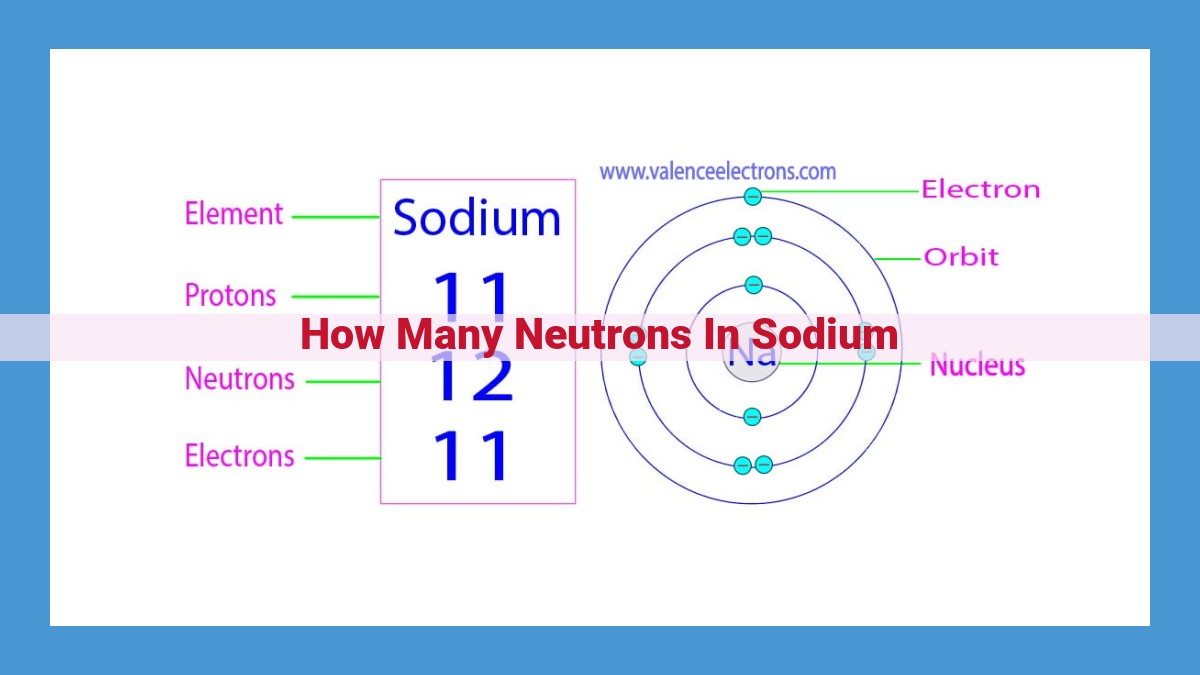
At the heart of every atom lies its nucleus, a tiny powerhouse containing protons and neutrons. Protons, positively charged particles, define an element’s identity. Sodium, with 11 protons, belongs to the group of alkali metals. Neutrons, on the other hand, carry no electrical charge and contribute to an atom’s mass.
Mass Number: Unraveling the Neutron Count
The total number of protons and neutrons in an atom’s nucleus is known as its mass number. This number provides a valuable clue to determine the neutron count. For sodium, the mass number is 23. To isolate the number of neutrons, we subtract the number of protons (11) from the mass number, revealing that sodium has 12 neutrons.
Isotopic Variations: Sodium’s Neutron Spectrum
Sodium exists in various isotopic forms, which are atoms of the same element with differing neutron counts. The most prevalent isotope is Sodium-23, with 12 neutrons, accounting for over 99% of naturally occurring sodium. However, other isotopes, such as Sodium-24, with 13 neutrons, also exist. These isotopes possess the same chemical properties but differ in their mass and certain physical behaviors.
Sodium-23: The Workhorse Isotope
Sodium-23, with its 11 protons, 11 electrons, and 12 neutrons, is the backbone of sodium’s chemistry. This stable isotope is responsible for the element’s characteristic properties, such as its high reactivity and silvery-white appearance. It plays a vital role in regulating fluid balance and maintaining nerve function in living organisms.
Sodium-24: A Radioactive Beacon for Applications
Sodium-24, with 12 neutrons, is a radioactive isotope of sodium that finds diverse applications in science and medicine. Its short half-life (15 hours) makes it useful for tracing biological processes, such as blood flow and sodium transport in the body. Additionally, Sodium-24 is employed in cancer treatment, where it targets and destroys cancerous cells with precision.
By unraveling the concepts of atomic number, mass number, and isotopes, we have illuminated the path to determining the number of neutrons in sodium. Understanding these fundamental principles provides a deeper appreciation for the intricacies of atomic structure and the fascinating world of chemistry that governs our lives.
Atomic Number: Unraveling the Identity of Sodium
In the realm of chemistry, understanding the fundamental building blocks of matter is crucial. One such key element is sodium, and determining the number of neutrons it possesses is essential for comprehending its nature. To unlock this mystery, we must delve into the concept of atomic number and its profound implications for sodium’s identity.
The atomic number of an element reveals the number of protons residing in its atomic nucleus. These protons, bearing a positive charge, define an element’s position on the periodic table. Sodium, with an atomic number of 11, proudly occupies the 11th position, indicating the presence of 11 protons within its nucleus.
Atomic number, therefore, serves as a unique fingerprint for each element, determining its chemical properties and behavior. Sodium’s atomic number of 11 signifies that every sodium atom contains 11 protons, irrespective of its neutron count. This distinctive characteristic forms the foundation of sodium’s chemical identity and governs its reactivity, bonding patterns, and position within the periodic table. Understanding the atomic number of sodium is the cornerstone of unraveling its unique characteristics and unlocking the secrets it holds.
Mass Number and Sodium’s Composition
Sodium is a crucial element that plays a significant role in various biological and chemical processes. Understanding its composition is essential to unravel its properties and behavior. One key aspect of sodium’s composition is its mass number, which provides valuable insights into the number of neutrons present within its atoms.
The mass number of an atom is defined as the sum of the number of protons and neutrons in its nucleus. In the case of sodium, its atomic number is 11, indicating the presence of 11 protons in its nucleus. As the number of electrons in a neutral atom is equal to the number of protons, sodium also has 11 electrons.
To determine the number of neutrons in sodium, we can utilize the formula: Mass Number = Number of Protons + Number of Neutrons. Substituting the known values, we get: Mass Number = 11 (Number of Protons) + Number of Neutrons. Rearranging the equation, we can solve for the Number of Neutrons: Number of Neutrons = Mass Number – Number of Protons.
Determining the Number of Neutrons in Sodium
The mass number of sodium is 23, which represents the total number of protons and neutrons in its nucleus. Since we know that sodium has 11 protons, we can simply subtract this value from the mass number to obtain the number of neutrons: Number of Neutrons = 23 (Mass Number) – 11 (Number of Protons) = 12 Neutrons.
Understanding the concept of mass number allows us to determine the number of neutrons in sodium, which is crucial for comprehending its composition and behavior. Sodium’s mass number of 23 and atomic number of 11 reveal that it possesses 12 neutrons in its nucleus, influencing its chemical and physical properties.
Isotopes of Sodium: Unraveling the Variations in Neutron Count
The world of chemistry is filled with fascinating elements, each with its unique characteristics. Sodium, an alkali metal known for its high reactivity, is no exception. One intriguing aspect of sodium is its existence in various isotopes, which are atoms of the same element that differ in their neutron count.
Defining Isotopes and Their Significance
Isotopes are essentially doppelgangers of the same element, sharing the same atomic number (number of protons) but differing in their mass number (number of protons plus neutrons). This variation in neutron count influences the mass and certain properties of the isotopes.
Sodium’s Isotopic Family
Sodium boasts an array of isotopes, each with a distinct neutron count. The most common isotope, Sodium-23, is the workhorse of the sodium family, accounting for over 99% of naturally occurring sodium. It contains 11 protons, 11 electrons, and 11 neutrons.
Unveiling Sodium-24: A Radioactive Isotope with Remarkable Applications
Among the sodium isotopes, Sodium-24 stands out with its 12 neutrons. This radioactive isotope possesses unique properties that make it invaluable in various scientific and medical fields. For instance, Sodium-24 is widely used in medicine as a tracer in biological processes and in the treatment of specific cancers.
Connecting the Dots: Determining Sodium’s Neutron Count
Understanding the concepts of atomic number, mass number, and isotopes is crucial for comprehending the neutron count of sodium. By combining these principles, we can unravel the specific neutron count of each sodium isotope, which in turn influences their properties and applications.
Sodium-23: The Abundant Isotope Shaping Sodium’s Properties
Among the various isotopes of sodium, Sodium-23 stands out as the predominant form, shaping the element’s fundamental characteristics and its significance in the chemical realm. With 11 protons, 11 electrons, and 11 neutrons in its atomic nucleus, Sodium-23 constitutes the majority of naturally occurring sodium.
The Foundation of Sodium’s Identity
The atomic number, a defining attribute of an element, reflects the number of protons within its nucleus. For sodium, this number is 11, establishing its identity as the 11th element on the periodic table. The atomic number directly influences the element’s chemical behavior and properties.
Mass Number and Neutron Count
The mass number, another crucial concept, represents the total number of protons and neutrons in the nucleus. Sodium-23 boasts a mass number of 23, indicating the presence of 11 protons and 11 neutrons. This configuration grants sodium-23 its characteristic properties and distinguishes it from other sodium isotopes.
Sodium’s Chemical and Physical Attributes
The abundance of sodium-23 profoundly influences sodium’s overall chemical and physical behavior. This isotope is responsible for the element’s high reactivity, flammability, and its vital role in biological processes. Sodium-23 plays a pivotal role in regulating fluid balance and nerve impulses in living organisms.
By understanding the atomic number, mass number, and isotope concept, we unravel the mystery of sodium’s neutron count. Sodium-23, the predominant isotope, forms the foundation of sodium’s identity and properties, shaping its interactions with other elements and its significance in the realm of chemistry. Unraveling the secrets of atomic structure empowers us to comprehend the intricate tapestry of the chemical world.
Sodium-24: A Radioactive Isotope with Applications
As we delve deeper into the fascinating world of sodium, we come across its radioactive isotope, Sodium-24. This unique form of sodium, boasting 12 neutrons, holds significant applications in medical advancements.
Uncovering the Properties of Sodium-24
Sodium-24 distinguishes itself by its radioactive nature. This inherent property allows it to emit energy in the form of radiation, which can be harnessed for various purposes. Its radioactive decay occurs with a half-life of 15 hours, meaning that half of the Sodium-24 atoms decay within this time frame.
Sodium-24’s Applications in Medicine
Harnessing the radioactive properties of Sodium-24, scientists have developed innovative medical applications:
-
Tracing Biological Processes: Sodium-24 can be introduced into the body via injection. As it circulates through the bloodstream, its radiation can be tracked and measured to reveal information about blood flow, organ function, and other physiological processes.
-
Treating Certain Cancers: Sodium-24’s radiation can be precisely targeted to cancerous tissues. By delivering a concentrated dose of radiation directly to the tumor, Sodium-24 can effectively destroy cancer cells while minimizing damage to surrounding healthy tissue. This approach is particularly beneficial for treating certain types of cancer, such as lymphoma and leukemia.
Sodium-24’s unique properties have transformed it into a valuable tool for medical research and treatments. Its ability to trace biological processes and target cancerous tissue underscores its significance in advancing the fight against disease.