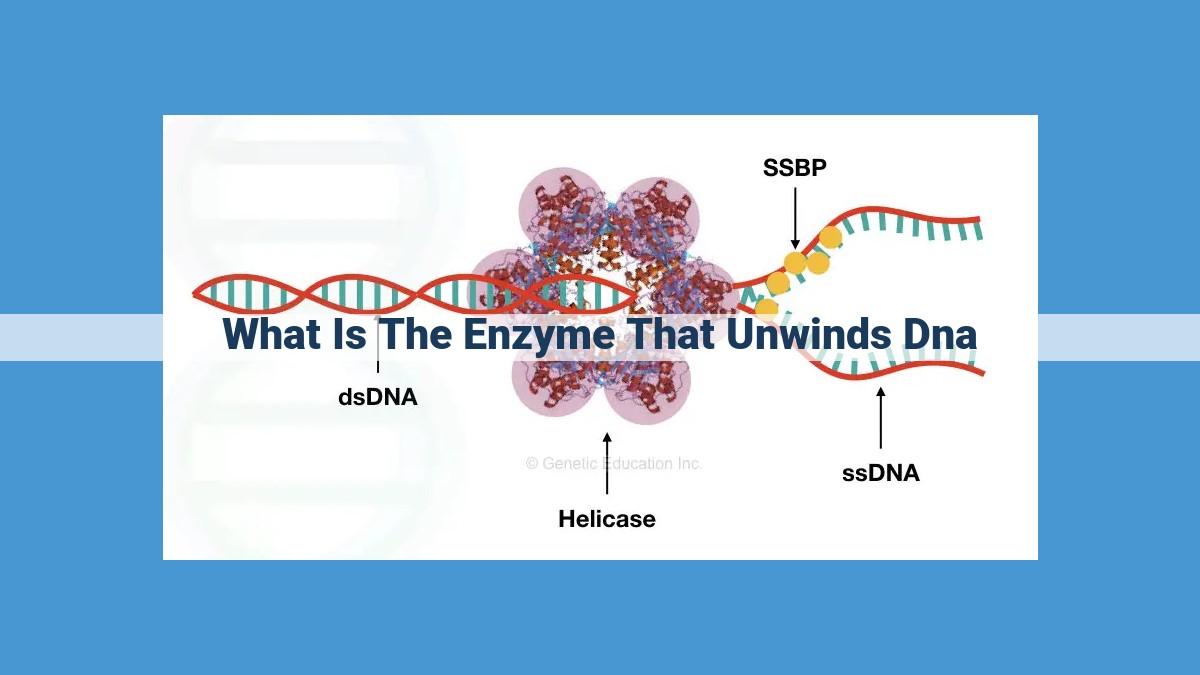
DNA Helicase, an enzyme vital for DNA replication, unwinds the DNA double helix by breaking hydrogen bonds between base pairs. It plays a crucial role in forming the replication fork and facilitates subsequent processes such as DNA synthesis and gene expression.
DNA Helicase: The Key to Unwinding DNA
- Definition of DNA helicase and its role in unwinding the DNA double helix.
- Importance of helicase in DNA replication and formation of the replication fork.
DNA Helicase: Unraveling the Secrets of DNA’s Replication
In the intricate dance of life, DNA replication stands as a crucial step, ensuring the faithful transmission of genetic information from one generation to the next. Central to this process is DNA helicase, an enzyme that plays the pivotal role of unwinding the tightly coiled DNA double helix, revealing the template strands for replication.
The Engine of Unwinding
Picture DNA as a twisted ladder with two strands forming the sides and connecting rungs. DNA helicase, like a molecular acrobat, maneuvers along the length of this ladder, deftly breaking the hydrogen bonds that hold the strands together. As it does so, the DNA unwinds, creating a replication fork – a Y-shaped region where the two strands of the double helix separate.
Importance in DNA Replication
This unwinding process is essential for DNA replication to occur. The replication fork provides a region where replication enzymes can access the DNA template and begin synthesizing new strands complementary to the existing ones. Without helicase’s action, the DNA would remain tightly bound, hindering replication and potentially disrupting the entire process.
Mechanism of Action
DNA helicase uses the energy derived from ATP hydrolysis to unwind the DNA double helix. It binds to the DNA at a specific site and begins to move along one strand. As it progresses, it breaks the hydrogen bonds between the bases, separating the strands. Helicase also utilizes other proteins, known as single-strand binding proteins, to stabilize the unwound DNA and prevent it from re-annealing.
DNA helicase is a fundamental enzyme in the process of DNA replication. Its ability to unwind the DNA double helix is essential for creating the replication fork and allowing replication enzymes to synthesize new DNA strands. By playing this crucial role, helicase ensures the accurate transmission of genetic information, laying the foundation for life’s continuity.
Replication: The Process of DNA Duplication
In the remarkable world of genetics, the duplication of DNA, the blueprint of life, is a fundamental process known as DNA replication. It’s a meticulously orchestrated symphony involving a cast of molecular players, each performing specific roles to ensure the faithful transmission of genetic information from one generation to the next.
DNA replication unfolds in three distinct stages: initiation, elongation, and termination. Initiation marks the beginning, where specific DNA sequences act as signals for replication to commence. Proteins called helicase come into play, acting like tiny molecular scissors, snipping apart the DNA double helix. This unwinding process creates a replication fork, the Y-shaped region where the actual copying takes place.
Elongation is the heart of the replication process. The unwound DNA serves as a template, directing the synthesis of new DNA strands. Specialized enzymes, DNA polymerases, are the master builders, using the template to add nucleotides, the building blocks of DNA. There’s a twist, though: because DNA is double-stranded, two new strands are synthesized simultaneously.
One strand, known as the leading strand, elongates continuously in the same direction as the replication fork moves. The other strand, the lagging strand, is built in fragments called Okazaki fragments. These fragments are later joined together by another molecular helper, DNA ligase.
The termination stage brings the replication process to an end. Specific sequences in the DNA signal the completion of replication. Once the final strands are synthesized and ligated, the newly replicated DNA molecules are identical copies of the original, ready to embark on their own genetic journeys.
Leading and Lagging Strands: Unveiling the Directional Dance of DNA Replication
In the intricate tapestry of DNA replication, the synthesis of new DNA strands unfolds in a fascinating dance of opposing directions. This interplay of leading and lagging strands unravels the secrets of duplicating our genetic blueprint with remarkable precision.
The Replication Fork: A Crossroads of Synthesis
Imagine a replication fork, a Y-shaped structure that forms as DNA helicase unwinds the parent double helix. At this molecular crossroads, the replication machinery sets in motion the synthesis of new DNA strands.
Leading Strand: A Smooth Journey Awaits
On the leading strand, DNA polymerase, the workhorse of replication, glides along the template strand in a continuous, seamless fashion. The newly synthesized daughter strand elongates steadily from the 5′ to 3′ end, matching the unwinding of the parental DNA.
Lagging Strand: A Discontinuous Synthesis Saga
Across the replication fork, the lagging strand presents a different narrative. Here, DNA polymerase faces a unique challenge: the replication fork moves in the opposite direction, leaving gaps in the unwound DNA. To overcome this obstacle, the lagging strand synthesis proceeds in short, fragmental bursts called Okazaki fragments.
Primase: The Guiding Spark
Primase, the initiator of DNA synthesis, plays a pivotal role in this discontinuous dance. It lays down short RNA primers on the lagging strand template, providing a temporary foothold for DNA polymerase. As the lagging strand elongates, RNase H swiftly removes the RNA primers, leaving gaps to be filled.
DNA Ligase: The Final Stitch
Enter DNA ligase, the molecular seamstress that joins the growing fragments together. With meticulous precision, it covalently bonds the Okazaki fragments, completing the synthesis of the lagging strand and forging a cohesive new DNA molecule.
Thus, the dance of leading and lagging strands continues, ensuring the accurate and timely duplication of our genetic material. This intricate interplay of directional synthesis lies at the heart of cell division, preserving the integrity of our DNA and passing on our hereditary traits to future generations.
Okazaki Fragments: The Building Blocks of the Lagging Strand
In the intricate dance of DNA replication, the continuous synthesis of the leading strand stands in contrast to the piecemeal creation of the lagging strand. This intricate process relies on a remarkable molecular machine known as the replisome, a complex of proteins that orchestrates the duplication of our genetic blueprint.
The Birth of Okazaki Fragments
The replisome initiates DNA replication from specific locations called origins of replication. As the DNA double helix unwinds, an enzyme called primase enters the fray, its task to lay down short RNA primers. These primers provide a starting point for DNA polymerases, the tireless workers that synthesize new DNA strands.
On the leading strand, the DNA polymerase marches forward continuously, reading the template strand and adding complementary nucleotides. The lagging strand, however, faces a unique challenge. The replication fork moves in the 5′ to 3′ direction, but DNA polymerase can only synthesize in the 3′ to 5′ direction. This conundrum is solved through the formation of Okazaki fragments.
The Assembly Line of Okazaki Fragments
Okazaki fragments are short, newly synthesized DNA segments that are created discontinuously on the lagging strand. Primase lays down an RNA primer, providing a toehold for DNA polymerase. As the polymerase extends the fragment, the primer is displaced, creating a gap.
The replisome contains another enzymatic helper, RNase H, which swiftly removes the RNA primers. The gaps between Okazaki fragments are then filled in by DNA polymerase, sealing the fragments together. This process continues until the lagging strand is fully synthesized.
The Importance of Okazaki Fragments
Okazaki fragments are essential for the replication of the lagging strand. Without them, the DNA polymerase would be unable to synthesize the lagging strand in a continuous manner. The discontinuous synthesis of Okazaki fragments ensures that the replication process can keep pace with the unwinding of the DNA double helix.
A Well-Oiled Machine
The formation and processing of Okazaki fragments highlight the amazing teamwork that takes place within the replisome. Primase, RNase H, and DNA polymerase coordinate their actions to ensure the accurate and efficient duplication of our genetic material. These molecular machines are essential for the very foundation of life, safeguarding the integrity of our DNA and ensuring the continuity of genetic information from one generation to the next.
Primase: The Unsung Hero of DNA Replication
In the intricate dance of DNA replication, there’s a player that often goes unnoticed but plays a crucial role: primase. This enzyme is the sparkplug that ignites DNA synthesis on the lagging strand, the counterpart to the more famous leading strand.
Primase is a specialized enzyme that synthesizes RNA primers, short segments of RNA that serve as a foundation for DNA replication. DNA polymerases, the workhorses of replication, can’t start building DNA from scratch; they need a primer to bind to and extend.
Primase’s action is essential because DNA replication occurs semi-discontinuously. On the leading strand, DNA is synthesized continuously in the same direction as the unwinding of the DNA helix. But on the lagging strand, synthesis must occur discontinuously in small fragments called Okazaki fragments because the helix unwinds and rewinds in the opposite direction.
Here’s how primase fits into the picture: as the DNA helicase unwinds the lagging strand, primase steps in and synthesizes short RNA primers at various points along the strand. These primers provide a foothold for DNA polymerases to bind and start adding DNA nucleotides, forming the Okazaki fragments.
Once the Okazaki fragments are complete, primase’s role is done. However, it leaves behind a trail of RNA primers that need to be removed for the DNA strands to become fully complete. That’s where RNase H, another specialized enzyme, comes in, chopping up the RNA primers so DNA polymerase can fill in the gaps, creating a continuous strand of DNA.
So, while primase may not be as flashy as some other players in the DNA replication process, its role is indispensable. It’s the one that initiates the synthesis of the lagging strand, ensuring that the entire DNA molecule is faithfully duplicated and ready to carry the blueprint of life forward.
DNA Polymerase: The Precision Engineer Behind DNA Replication
DNA replication is a fundamental process that ensures the accurate transmission of genetic information from one generation of cells to the next. At its heart lies _DNA polymerase, _a molecular virtuoso that catalyzes the synthesis of new DNA strands with remarkable precision.
Types and Mechanisms
There are various types of DNA polymerases, each with a specific role in the replication process:
- DNA Polymerase III: This is the main polymerase involved in continuous, uninterrupted synthesis on the leading strand.
- DNA Polymerase I: This enzyme primarily performs discontinuous, fragment-based synthesis on the lagging strand.
- DNA Polymerase II: It participates in DNA repair and fills in gaps where DNA synthesis was interrupted.
Template-Directed Synthesis
DNA polymerase operates on a principle of template-directed synthesis, meticulously copying the template strand of DNA. It reads the sequence of nucleotides on the template and inserts complementary nucleotides into the newly synthesized strand. This ensures the accurate duplication of genetic information.
Proofreading Capabilities
DNA polymerase is not just a copycat; it’s also a meticulous proofreader. It possesses an exonuclease activity that allows it to check and correct errors that may arise during nucleotide incorporation. This proofreading step ensures the high fidelity of DNA replication, minimizing the accumulation of harmful mutations.
DNA polymerase is the heart of the DNA replication machinery, a molecular artist that weaves new DNA strands with precision and care. Its types, mechanisms, and proofreading capabilities combine to guarantee the integrity and accuracy of genetic information, ensuring the continuity of life and the preservation of our genetic heritage.
RNase H: The Unsung Hero of DNA Replication
In the intricate dance of DNA replication, RNase H plays a pivotal role, removing the RNA primers that initiate DNA synthesis on the lagging strand. It’s a tale of precision and teamwork, ensuring the integrity of our genetic code.
RNase H is an enzyme that specifically targets and cleaves RNA molecules. In DNA replication, it has the delicate task of removing the RNA primers synthesized by primase to initiate DNA synthesis on the lagging strand. These primers are temporary placeholders, marking the starting points for DNA polymerase to build new DNA strands.
Once DNA polymerase elongates the new DNA strand, RNase H follows closely behind, scanning for the RNA primers. When it encounters an RNA-DNA junction, it cleaves the RNA strand, leaving a gap in the DNA. This gap is then filled by DNA polymerase, which incorporates deoxyribonucleotides to complete the new DNA strand.
The specificity of RNase H is crucial to prevent it from damaging the newly synthesized DNA. It recognizes the RNA-DNA junction through specific structural features, ensuring that it only targets the primers and not the DNA itself. This specificity is essential for maintaining the integrity of our genetic information.
The removal of RNA primers by RNase H is a critical step in the completion of DNA replication. Without this enzyme, the lagging strand would remain incomplete, compromising the replication process and potentially leading to errors in DNA synthesis. Thus, RNase H, though often overlooked, is an indispensable player in the intricate machinery of DNA replication.
DNA Ligase: Sealing the Gaps in DNA Replication
In the intricate dance of DNA replication, where the genetic blueprints of life are duplicated with meticulous precision, there’s a crucial player that holds the power to seal the gaps and complete the masterpiece: DNA ligase.
Imagine a construction site where a team of skilled builders erects a magnificent structure. But the job isn’t done until the last brick is placed and the final mortar joins the pieces together. DNA ligase is that indispensable mortar, the master craftsman who brings the newly synthesized DNA strands into perfect alignment and binds them together.
On the lagging strand, where DNA synthesis occurs in short segments known as Okazaki fragments, DNA ligase plays a pivotal role. These fragments, like tiny jigsaw puzzle pieces, must be covalently joined to form a continuous and cohesive strand.
The Mechanism of Action:
With its molecular toolbox, DNA ligase meticulously scans the Okazaki fragments, searching for their complementary ends. Once it identifies a suitable match, it deftly joins the 3′-hydroxyl group of one fragment to the 5′-phosphate group of the other. This exquisite bond, known as a phosphodiester bond, is the cornerstone of DNA’s double-helix structure.
The Importance of DNA Ligase:
DNA ligase’s role is not to be underestimated. Without its impeccable craftsmanship, the newly synthesized DNA would remain fragmented and unstable. The integrity of the genetic code would be compromised, potentially leading to errors and mutations.
Furthermore, DNA ligase provides a safety mechanism, safeguarding the genome from structural defects. By ensuring that all Okazaki fragments are securely joined, it prevents gaps or nicks that could disrupt the vital processes of transcription and translation.
In conclusion, DNA ligase is the unsung hero of DNA replication, the indispensable enzyme that seals the gaps and ensures the flawless transmission of genetic information. Its remarkable ability to stitch together the building blocks of life is a testament to the intricate beauty and resilience of our genetic code.