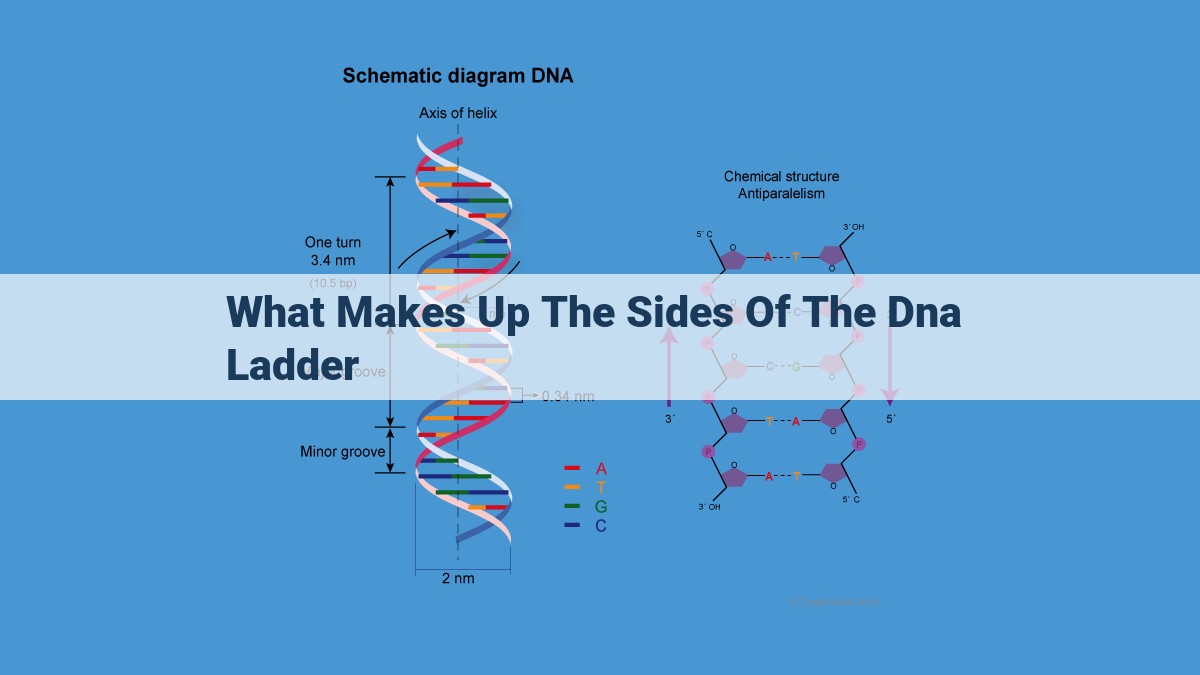
The sides of the DNA ladder are formed by base pairs, which are complementary pairs of nitrogenous bases. These bases, adenine (A), thymine (T), cytosine (C), and guanine (G), always pair up in the same way according to specific base pairing rules (A-T, C-G). These base pairs, connected by hydrogen bonds, create the rungs of the “ladder”. The rails of the ladder consist of the sugar-phosphate backbone, a repeating chain of alternating deoxyribose sugar and phosphate groups, providing structural support and stability to the DNA molecule.
Decoding the Blueprint of Life: Exploring the Building Blocks of DNA
Imagine a tiny molecular world where the secrets of life are encoded in the intricate structure of a molecule called DNA. At its core, DNA consists of fundamental building blocks known as nucleotides. Picture these nucleotides as the individual bricks that assemble the vast blueprint of all living organisms.
Understanding Nucleotides: The Essential Triad
Each nucleotide is a small molecule composed of three essential components:
-
A sugar molecule (deoxyribose): The sugary backbone that forms the structural scaffold of DNA.
-
A phosphate group (PO4): The negatively charged appendage that connects nucleotides to form a linear chain.
-
A nitrogenous base: The crucial component responsible for the genetic information encoded within DNA.
The Nitrogenous Bases: The Alphabet of Genetics
The nitrogenous bases are the alphabet of genetics, providing the distinct letters that spell out the genetic code. There are four main bases in DNA:
- Adenine (A): A purine base with a double-ring structure.
- Thymine (T): A pyrimidine base with a single-ring structure.
- Cytosine (C): A pyrimidine base with a single-ring structure.
- Guanine (G): A purine base with a double-ring structure.
The Intriguing Game of Base Pairing
The nitrogenous bases have a remarkable ability to pair with each other through hydrogen bonds. This pairing follows specific rules:
- Adenine always pairs with thymine through two hydrogen bonds.
- Cytosine always pairs with guanine through three hydrogen bonds.
Building the DNA Ladder: Complementary Strands
The base pairing rules allow for the formation of complementary strands of DNA. Each strand acts as a mirror image of the other, with the bases on one strand pairing with their complementary counterparts on the opposite strand. These paired strands twist together, forming the iconic double helix structure of DNA.
The Double Helix: A Twisted Ladder of Life
The double helix is a stunning molecular marvel, resembling a twisted ladder. The paired bases form the rungs of the ladder, while the sugar-phosphate backbone acts as the sturdy rails, providing structural support and stability.
In Summary
Nucleotides, the building blocks of DNA, are composed of sugar, phosphate, and nitrogenous bases. The four nitrogenous bases (A, T, C, and G) pair with each other to form complementary DNA strands. These strands then intertwine to create the double helix, the iconic structure that holds the genetic blueprints for all living organisms.
The Four Building Blocks of DNA: Adenine, Thymine, Cytosine, and Guanine
As we delve into the intricate world of DNA, the blueprint of life, we encounter four fundamental building blocks known as nucleotides. Each nucleotide comprises three essential components: a sugar molecule, a phosphate group, and a nitrogenous base. The nitrogenous bases, in particular, play a pivotal role in the unique structure of DNA.
There are four types of nitrogenous bases in DNA: adenine, thymine, cytosine, and guanine. These bases are like the alphabet of DNA, and they pair up with each other in a specific manner to form the rungs of the DNA ladder. Adenine always pairs with its complementary base, thymine, while cytosine pairs with guanine. This pairing is known as complementary base pairing, and it’s essential for maintaining the stability and function of DNA.
The specific base pairing rules (A-T and C-G) ensure that the two strands of DNA run in opposite directions, forming a double helix structure. The double helix resembles a twisted ladder, where the base pairs form the rungs and the sugar-phosphate backbone acts as the rails. The hydrogen bonds between the base pairs provide structural stability to the DNA molecule, preventing it from unraveling or breaking apart.
In summary, the four nitrogenous bases—adenine, thymine, cytosine, and guanine—are responsible for the unique structure and complementary base pairing that define DNA. These bases form the rungs of the DNA ladder, ensuring the genetic information is accurately transmitted and maintained. Understanding the role of these bases is crucial for comprehending the fundamental principles of DNA and its central role in the inheritance of life.
Base Pairing: The Complementary Strands of the DNA Ladder
Picture DNA as a twisted, ladder-like structure. Each rung of this ladder is made up of base pairs. These base pairs are the fundamental units that hold the two strands of DNA together and allow for the storage and transmission of genetic information.
The bases that make up these pairs are not just any random building blocks; they follow strict pairing rules: adenine (A) always pairs with thymine (T), and cytosine (C) always pairs with guanine (G). This specific pairing is due to the chemical properties of each base, which allow them to form hydrogen bonds with each other. Hydrogen bonds are weak chemical bonds that form between electronegative atoms and hydrogen atoms, stabilizing the structure of the DNA molecule.
Two hydrogen bonds form between A and T, while three hydrogen bonds form between C and G. This difference in hydrogen bonding strength contributes to the stability of the DNA double helix, as the stronger bonds between C and G help to hold the structure together more tightly.
The complementary nature of base pairing ensures that the information encoded in one strand of DNA can be accurately copied onto the other. When a new strand is synthesized, the A on the original strand attracts a T, the T attracts an A, the C attracts a G, and so on. This pairing process creates a complementary strand that carries the same genetic information as the original.
In summary, base pairing is the foundation of DNA’s double helix structure and the key to its ability to store and transmit genetic information. By following specific pairing rules, the nucleotides that make up DNA form complementary strands that maintain the integrity and stability of the molecule, ensuring the accurate transmission of genetic traits from one generation to the next.
The Double Helix: Unraveling DNA’s Iconic Structure
Nestled within the microscopic realm of our cells lies a remarkable molecule, DNA, the blueprint of life. DNA’s intricate structure, akin to a twisted ladder, plays a pivotal role in storing and transmitting genetic information.
Imagine a spiral staircase, its individual steps represented by the base pairs of DNA. These base pairs, composed of nitrogenous bases, are like molecular cogs that fit together in precise pairings: adenine (A) always bonds with thymine (T), and cytosine (C) with guanine (G). These pairings form the sides of the DNA ladder, creating a complementary double-stranded molecule.
The rails of the ladder are formed by the sugar-phosphate backbone, a sturdy framework made up of alternating sugar and phosphate groups. The sugar-phosphate backbone provides structural support and stability, ensuring the integrity of the DNA molecule.
This iconic double helix structure not only protects DNA from damage but also allows it to be replicated during cell division. The strands can unzip like a zipper, allowing new complementary strands to be synthesized, ensuring the faithful transmission of genetic information.
By unraveling the double helix, we gain a deeper appreciation for the intricate machinery of life. DNA’s structure, with its precise base pairing and sturdy backbone, is a testament to the elegance of nature’s design.
The Sugar-Phosphate Backbone: The Unseen Framework of the DNA Ladder
Imagine a delicate ladder, its sides adorned with a harmonious dance of base pairs, the rungs that hold the structure together. But beneath this intricate display lies an unseen framework, a backbone that provides the DNA molecule with its stability and resilience: the sugar-phosphate backbone.
The sugar-phosphate backbone is composed of alternating units of deoxyribose sugar and phosphate groups. The deoxyribose sugar is a five-carbon sugar, while the phosphate group is a negatively charged molecule. The sugar and phosphate groups are linked together by phosphodiester bonds, forming a chain that runs along the sides of the DNA molecule.
This backbone is not merely a passive support structure. It plays a vital role in maintaining the DNA’s stability. The negative charges of the phosphate groups repel each other, creating a repulsive force that prevents the DNA strands from collapsing in on themselves. This repulsive force is further reinforced by the hydration shell, a layer of water molecules that surrounds the phosphate groups. The water molecules interact with the phosphate groups, forming hydrogen bonds that stabilize the DNA structure.
In addition to its structural role, the sugar-phosphate backbone also provides a chemical framework for the DNA molecule. The deoxyribose sugar contains a carbon atom at position 2′, which is linked to a hydrogen atom in double-stranded DNA. This hydrogen atom participates in hydrogen bonding with the nitrogenous base of the opposite strand, helping to stabilize the base pairs.
The sugar-phosphate backbone is an essential component of DNA, providing it with the strength and stability it needs to carry out its crucial biological functions. Without this unseen framework, the DNA molecule would be fragile and unable to withstand the rigors of cellular life.