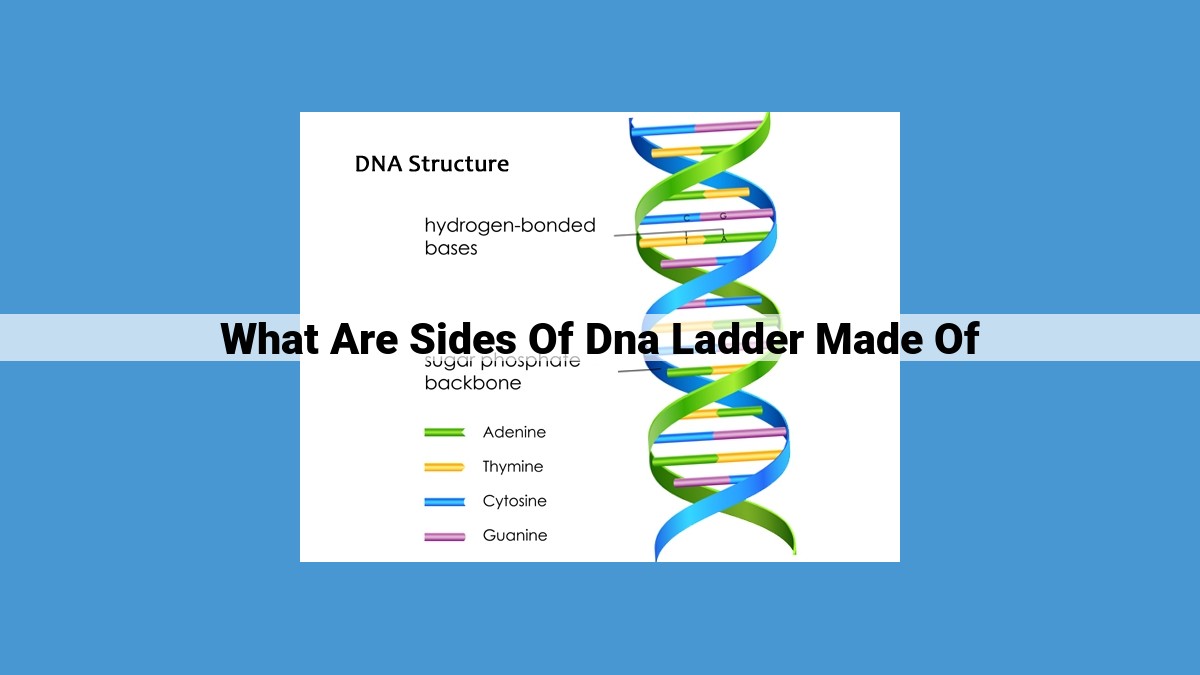
The sides of the DNA ladder consist of alternating deoxyribose sugars and phosphate molecules, forming a sugar-phosphate backbone. Deoxyribose, a five-carbon sugar, provides the structural framework, while phosphate groups, negatively charged ions, connect the sugars via phosphodiester bonds. Together, they create the rigid backbone that forms the sides of the DNA double helix.
The Sugar-Phosphate Backbone: The Framework of DNA
Imagine a ladder that carries the blueprint of life – that’s DNA. The rungs of this ladder are pairs of nitrogenous bases, but the framework that holds it all together is the sugar-phosphate backbone.
This backbone is made up of deoxyribose, a five-carbon sugar, and phosphate, a negatively charged molecule. The deoxyribose molecules form the sides of the ladder, while the phosphate molecules connect them like rungs.
Each deoxyribose molecule has a carbon atom numbered 1′ to 5′. The phosphate molecule attaches to the 5′ carbon of one deoxyribose and the 3′ carbon of the next, creating a linear chain.
This chain is polar, meaning it has a positive end (the 5′ end) and a negative end (the 3′ end). This polarity is crucial for DNA replication and transcription, where the DNA strands can be recognized and copied.
Deoxyribose: The Pentose Sugar in DNA’s Blueprint
Picture this: DNA, the blueprint of life, is a double helix ladder-like structure. The backbone of this sturdy ladder, the framework that holds everything together, is made of two key components – deoxyribose and phosphate.
Deoxyribose is a pentose sugar, meaning it has five carbon atoms. It’s the core sugar molecule that gives DNA its distinctive shape. Deoxyribose forms the vertical sides of the DNA ladder, while the phosphate molecules connect these sides, forming the horizontal rungs.
The chemical bonds involved in deoxyribose’s role are covalent bonds. These bonds are formed when two atoms share electrons, creating a strong connection. In DNA, deoxyribose molecules form covalent bonds with phosphate groups, creating a sugar-phosphate backbone. This backbone is the very foundation of DNA’s double helix structure.
Without deoxyribose, DNA would essentially collapse. It would lose its ladder-like shape and its ability to store genetic information. Deoxyribose’s role as the backbone of DNA is essential for life as we know it.
Phosphate: The Negatively Charged Side Groups
The DNA backbone, the scaffold of our genetic blueprint, is a marvel of molecular architecture. Connecting the sugar-phosphate backbone are phosphate molecules, the negatively charged side groups that impart stability and functionality to the DNA molecule.
Phosphate ions (PO43-) are composed of a central phosphorus atom surrounded by four oxygen atoms. This arrangement gives phosphate ions a tetrahedral shape with four negatively charged oxygen atoms. These negative charges are crucial for the DNA backbone’s stability.
The phosphate molecules line up along the sides of the DNA ladder, forming the “steps” of the double helix. Phosphate groups from opposite strands interact with each other, creating a strong electrostatic bond that holds the two strands together. This interaction is essential for the stability and integrity of the DNA molecule.
The acidic nature of the DNA backbone is another consequence of the presence of phosphate groups. The negative charges on the phosphate groups create an acidic environment, which has important physiological implications. For example, it helps to neutralize the positively charged cations in the cell, maintaining the proper pH balance.
The acidic nature of DNA also plays a role in gene regulation. Changes in pH can affect the accessibility of DNA to transcription factors, which are proteins that bind to specific DNA sequences to initiate gene expression. By altering the pH, cells can fine-tune gene expression, controlling which genes are turned on or off.
In summary, phosphate molecules are essential components of the DNA backbone, providing stability and functionality. Their negative charges contribute to the acidic nature of DNA, which has important implications for gene regulation and cellular physiology. Understanding the role of phosphate groups is crucial for comprehending the structure and function of DNA, the blueprint of life.
Nucleotide Subunits: The Building Blocks of DNA
In the realm of genetics, the blueprint of life lies within the enigmatic molecule known as DNA. This remarkable structure, the double helix, holds the secrets to our genetic heritage, controlling everything from eye color to susceptibility to disease. At the heart of this intricate molecule lie nucleotide subunits, the fundamental units that assemble to form the information-rich DNA strands.
Each nucleotide is a meticulously crafted trio, composed of three essential components:
- Deoxyribose: A five-carbon sugar that forms the structural backbone of the DNA double helix.
- Phosphate: A negatively charged molecule that forms the sides of the DNA ladder, connecting the deoxyribose molecules and giving the molecule its overall acidic nature.
- Nitrogenous Base: A ring-structured molecule that comes in four distinct types: adenine, thymine, guanine, and cytosine. These bases pair up, forming the infamous “rungs” of the DNA ladder that carry the genetic code.
These nucleotide subunits are not mere passive building blocks; they play an active role in the symphony of life. They orchestrate the replication of genetic information, facilitating cell division and the passing of traits from generation to generation. Additionally, modifications to these nucleotides, such as methylation, can influence gene expression, adding another layer of complexity to the genetic dance.
The four nitrogenous bases – adenine, thymine, guanine, and cytosine – form the basis of the genetic code. Adenine and thymine pair up, while guanine and cytosine make their companions. This specific pairing, known as complementary base pairing, is the very foundation of DNA’s ability to store and transmit information.
Understanding the structure and function of nucleotide subunits is not only essential for comprehending the mechanics of genetics but also provides insights into the very nature of life itself. By unraveling the mysteries of DNA’s building blocks, we gain a deeper appreciation for the intricate tapestry of genetic inheritance that shapes our existence.
Nucleosides: The Building Blocks of DNA
In the intricate tapestry of life, DNA stands as the blueprint that governs our genetic inheritance. At its very core lies a remarkable molecule known as deoxyribonucleic acid. This complex structure, shaped like a twisted ladder, is composed of nucleotides, the fundamental units of DNA.
Nucleosides are the building blocks that make up nucleotides. Each nucleoside consists of a deoxyribose sugar molecule bonded to a nitrogenous base. Deoxyribose is a five-carbon sugar that forms the backbone of the DNA double helix, while the nitrogenous bases are essential for carrying genetic information.
There are five types of nitrogenous bases found in DNA: adenine (A), thymine (T), guanine (G), cytosine (C), and uracil (U). Adenine and thymine are purines, characterized by a double-ring structure. Guanine, cytosine, and uracil are pyrimidines, featuring a single-ring structure.
Nucleosides play a crucial role in the synthesis and modification of DNA. During DNA replication, nucleosides are added to the growing DNA strand in a specific order, mirroring the nitrogenous base pairing rules: A pairs with T, and G pairs with C. This precise pairing ensures the faithful transmission of genetic information from one generation to the next.
Moreover, nucleosides are involved in various DNA repair mechanisms. When DNA is damaged by environmental factors or cellular processes, nucleosides can be used to replace damaged sections of DNA, ensuring the integrity of the genetic code.
In summary, nucleosides are essential components of DNA, acting as the basic building blocks that form nucleotides. Their role in DNA replication and repair is vital for maintaining the integrity of our genetic material and the proper functioning of cells and organisms. Understanding the structure and function of nucleosides provides a deeper appreciation for the remarkable complexity and elegance of life’s blueprint.