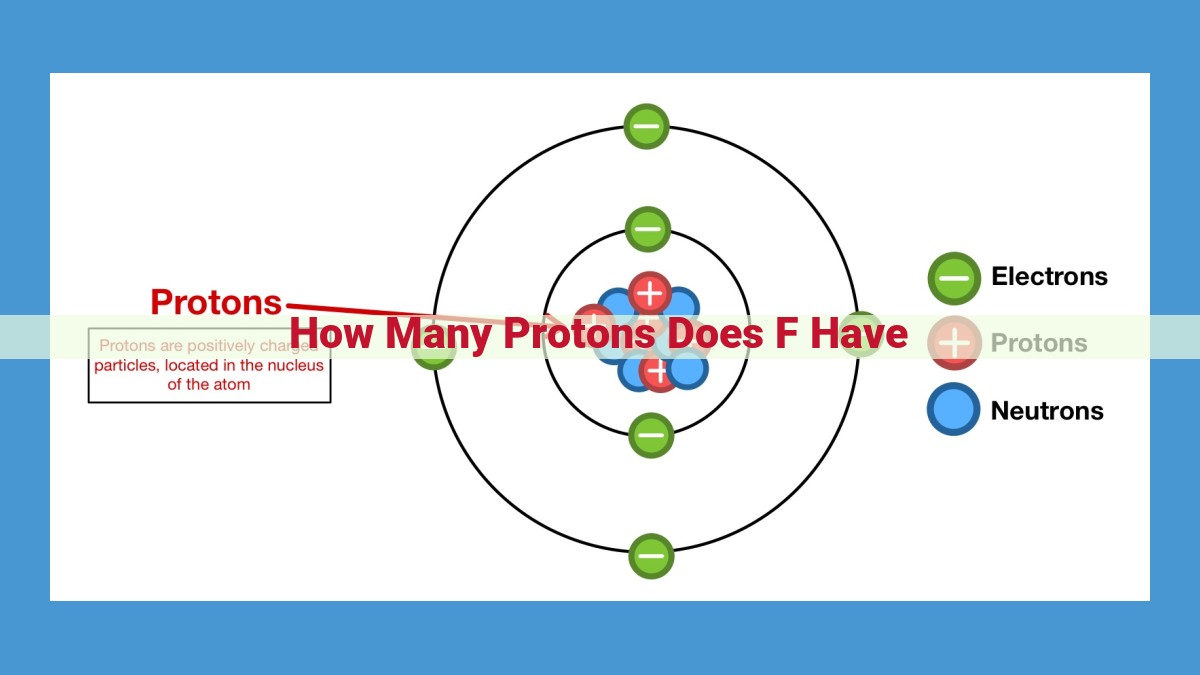
Protons, fundamental building blocks of atoms, contribute to an element’s identity and chemical properties. Fluorine (F), an element on the periodic table, has an atomic number of 9, indicating the presence of 9 protons in its nucleus. This atomic number defines fluorine’s position in the periodic table and influences its reactivity. Determining the number of protons in fluorine is crucial, as it shapes the element’s chemical behavior and allows for precise element classification and analysis in various scientific disciplines.
Protons: The Building Blocks of Matter
In the intricate world of chemistry, protons reign supreme as the fundamental particles that define the very essence of atoms. They reside at the heart of an atom’s nucleus, playing a crucial role in determining the element’s identity and behavior. In this captivating journey, we’ll embark on a voyage to unravel the fascinating story of protons, with a particular focus on the enigmatic element fluorine.
Atomic Number: Unveiling the Element’s Identity
Imagine a unique fingerprint that distinguishes each element from its brethren on the periodic table. This fingerprint, known as the atomic number, reveals the number of positively charged protons nestled within the nucleus. For instance, fluorine, symbolized by the letter “F,” boasts an atomic number of 9, indicating the presence of nine protons in its atomic core.
Fluorine’s Profile: A Chemical Enigma
Fluorine, an alluring element residing in Group 17 of the periodic table, exudes a pale yellow-green hue. It’s the lightest halogen, known for its remarkable reactivity and electronegativity. This enigmatic element forms the backbone of various compounds, including the widely used fluorspar (calcium fluoride), which finds applications in optics, metallurgy, and glass production.
Protons in Fluorine: Delving into the Core
At the heart of every fluorine atom lies a nucleus teeming with nine positively charged protons. This fundamental property distinguishes fluorine from other elements and profoundly influences its chemical behavior. The number of protons, along with the number of electrons, determines an element’s identity and position on the periodic table.
Chemical Properties: A Dance of Protons
The number of protons in fluorine not only defines its atomic number but also shapes its chemical properties. Fluorine’s nine protons create a strong pull on its electrons, making it the most electronegative element. This characteristic endows fluorine with a ravenous appetite for electrons, driving it to form covalent bonds with other atoms.
Calculating Protons in Fluorine: A Simple Equation
Determining the number of protons in a fluorine atom is a straightforward task. Simply recall its atomic number of 9. This number represents the unyielding foundation of protons that reside within the fluorine nucleus, forever defining its elemental identity.
As we delve deeper into the intricacies of chemistry, we recognize the profound significance of protons. They serve as the cornerstones of atomic structure, dictating the very essence of each element. By understanding the concept of atomic number, we gain the power to decipher the fingerprint of each element and uncover the secrets that lie within the atomic realm.
The Atomic Number: A Key to Understanding the Elements
In the vast realm of chemistry, the atomic number stands as a fundamental concept that unravels the secrets of chemical elements. Atomic number is the number of protons found in the nucleus of an atom, serving as a unique identifier for each element on the periodic table.
Understanding the Atomic Number
Imagine if every element was like a fingerprint, with a unique set of characteristics that set it apart from all others. The atomic number acts as the defining feature that bestows this individuality upon each element. It determines the number of protons, which in turn influences the number of electrons and the overall chemical properties of an element.
Fluorine: A Case in Point
Let’s take fluorine (F) as an example. This highly reactive element, nestled in Group 17 of the periodic table, has an atomic number of 9. This means that every fluorine atom possesses nine protons in its nucleus. This key piece of information not only distinguishes fluorine from other elements but also governs its unique chemical behavior.
The Influence of Protons
The number of protons within an atom profoundly impacts its chemical identity. Protons carry a positive electric charge, which attracts the negatively charged electrons orbiting the nucleus. The balance between protons and electrons dictates the element’s chemical reactivity, determining how it interacts with other atoms to form compounds.
In the case of fluorine, its nine protons create a strong electrostatic attraction for electrons, making it highly electronegative. This characteristic drives fluorine’s tendency to form chemical bonds with other elements, such as its well-known reactivity with hydrogen to form hydrogen fluoride.
Calculating Protons in an Atom
Determining the number of protons in an atom is a straightforward process. Simply refer to the element’s atomic number, which is typically found on the periodic table. In our fluorine example, the atomic number of 9 directly indicates the presence of nine protons in its nucleus.
The atomic number serves as a cornerstone of chemistry, providing a fundamental understanding of the elements that make up our world. By grasping this concept, we can unlock the secrets of chemical behavior and delve deeper into the intricacies of the periodic table. Remember, the atomic number is not just a number; it is the key that unlocks the doors to understanding the chemical universe.
Fluorine (F): The Unique Element with Abundant Protons
Protons, the positively charged particles within an atom’s nucleus, play a crucial role in shaping the fundamental characteristics of every element. Understanding their importance is vital in unraveling the mysteries of our chemical world.
Atomic Number and Chemical Identity
The atomic number of an element, a unique identifier, determines the number of protons in its nucleus. It represents the cornerstone upon which the Periodic Table is built, organizing elements into their families.
Introducing Fluorine (F)
Fluorine, an element denoted by the symbol F, resides in Group 17 of the Periodic Table, known as the halogens. Its atomic number of 9, the key to its identity, reveals intriguing insights into its structure and behavior.
Protons in Fluorine
With an atomic number of 9, fluorine atoms house precisely 9 protons in their nuclei. This knowledge is fundamental in understanding fluorine’s chemical properties and reactivity.
Exceptional Chemical Properties
The number of protons in fluorine influences its exceptional chemical properties. Fluorine exhibits high electronegativity and vigorously reacts with other elements, often forming stable compounds known as fluorides. Its intense reactivity makes it an essential component in various industries, including medicine and material science.
Calculating Protons in Fluorine
Determining the number of protons in a fluorine atom is straightforward. Simply refer to its atomic number on the Periodic Table, which is 9, indicating the presence of 9 protons in its nucleus.
Protons in Fluorine: Unraveling the Heart of an Element
In the vast tapestry of the universe, atoms form the very fabric of matter. Protons, the positively charged particles residing in the nucleus of an atom, play a crucial role in determining the identity and behavior of every element. Let’s embark on a journey to delve into the world of protons and unravel their significance in understanding the remarkable element, fluorine.
Fluorine, with its symbol “F”, is a highly reactive and electronegative gas that belongs to Group 17 on the periodic table. Its atomic number, a unique identifier for each element, is 9. This atomic number holds the key to understanding the number of protons in a fluorine atom.
The atomic number of an element dictates the number of positively charged protons within its nucleus. In the case of fluorine, its atomic number of 9 indicates that each fluorine atom contains 9 protons. These protons are tightly bound together by the strong nuclear force, forming the positively charged core of the atom.
The number of protons in an element’s nucleus determines its chemical identity. Each element has a specific number of protons, which distinguishes it from all other elements. For instance, hydrogen has 1 proton, helium has 2 protons, and oxygen has 8 protons.
Comprehending the concept of protons and atomic numbers is essential for understanding the periodic table and the intricate world of chemical interactions. By unraveling the secrets of protons, we gain a deeper appreciation for the fundamental building blocks of matter and the fascinating dance they perform in the vast expanse of the universe.
Fluorine: Unraveling the Secrets of a Reactive Element
Chemical Properties of Fluorine
In the realm of chemistry, the number of protons in an element’s nucleus plays a pivotal role in shaping its chemical properties. Fluorine (F), with its atomic number of 9, epitomizes this phenomenon. Let’s delve into how this crucial number influences the identity and behavior of this fascinating element.
Electronegativity: The Stellar Thief
The number of protons in fluorine’s nucleus makes it highly electronegative. This means it has a strong tendency to attract electrons from other atoms, creating a magnetic pull that makes it a relentless electron hog. This trait gives fluorine its characteristic reactivity and ability to form strong bonds.
Reactivity: A Dance of Partnerships
Fluorine’s high electronegativity makes it highly reactive, eager to participate in chemical reactions. It reacts with almost every element on the periodic table, except for the noble gases. This reactivity stems from its relentless pursuit of completing its outermost electron shell, a quest that drives it to form chemical bonds.
Chemical Identity: Unveiling the True Nature of Fluorine
The number of protons in fluorine’s nucleus defines its chemical identity. It determines the element’s position on the periodic table and its bonding characteristics. Fluorine belongs to Group 17, also known as the halogens, a family of highly reactive non-metals. This classification underscores its affinity for electrons and its exceptional reactivity.
The number of protons in fluorine’s nucleus is the keystone to understanding its chemical properties and identity. Its high electronegativity and reactivity make it a formidable force in the world of chemistry. By unraveling the secrets of fluorine’s atomic structure, we gain a deeper appreciation for the intricate interplay between protons and the chemical behavior of elements.
How to Calculate Protons in Fluorine: A Step-by-Step Guide
In the world of chemistry, understanding the fundamental building blocks of atoms is crucial. One of these crucial components is protons, which reside in the atomic nucleus and play a pivotal role in shaping the properties of an element. In this article, we will embark on a journey to discover how to calculate the number of protons in fluorine, an element known for its unique chemical characteristics.
Understanding Atomic Number
Before we delve into calculating protons, we must first grasp the concept of atomic number. This number, represented by the symbol Z, is the defining characteristic of an element and resides in the Periodic Table. It signifies the number of protons found within the nucleus of an atom. Each element possesses a distinct atomic number, making it a unique entity in the vast tapestry of chemical elements.
Fluorine: A Chemical Overview
Fluorine, denoted by the symbol F, is a highly reactive nonmetal that belongs to Group 17 of the Periodic Table. Its existence is characterized by its ability to form strong bonds with other elements, leading to its wide-ranging applications in industries such as medicine and manufacturing.
Protons in Fluorine
The atomic number of fluorine is 9. This number serves as a direct indication of the number of protons present in the nucleus of a fluorine atom. Since atomic number is a defining characteristic of an element, the number of protons is an immutable feature of fluorine.
Calculating Protons in Fluorine
Determining the number of protons in a fluorine atom is a straightforward process. By simply referencing the atomic number of fluorine (9), we can conclude that each fluorine atom contains 9 protons. This understanding provides a foundation for comprehending the chemical behavior of fluorine and its interactions with other elements.
In essence, understanding the number of protons in an element is crucial for deciphering its chemical properties. In the case of fluorine, its atomic number of 9 directly translates to 9 protons residing in the nucleus of each fluorine atom. This knowledge opens the door to exploring the fascinating world of chemical reactions and the intricate interplay of elements that shape our world.