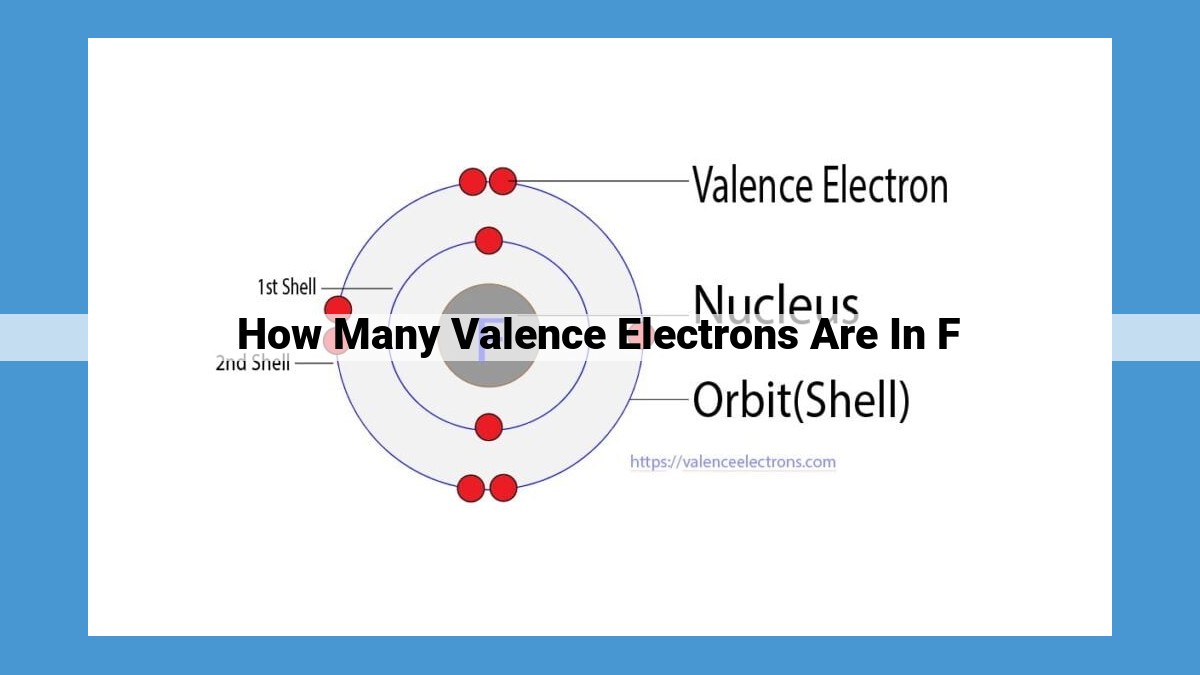
The F element, a halogen located in Group 17, possesses seven valence electrons. These electrons reside in the outermost energy level (2p orbital) of its electron configuration (1s²2s²2p⁵). Valence electrons play a crucial role in chemical bonding, as they determine the reactivity and bonding properties of an element. In F, the seven valence electrons allow it to form one covalent bond per atom by sharing these electrons with other atoms, resulting in the formation of various compounds and reactions.
What are Valence Electrons?
- Definition of valence electrons
- Role in chemical bonding
- Relationship to electron configuration
What Are Valence Electrons?
In the realm of chemistry, valence electrons are like the social butterflies of an atom. They’re the outermost electrons in an atom’s shell, eager to interact with the world. These electrons play a crucial role in chemical bonding, the dance that atoms perform to create molecules.
Just as our personalities shape our relationships, an atom’s valence electrons determine its chemical behavior. They’re the gateway to the atomic world, mediating interactions between atoms and governing the types of bonds they form.
Moreover, valence electrons are intimately linked to electron configuration, the arrangement of electrons within an atom. The number and position of valence electrons in an atom’s outermost shell dictate its chemical properties.
Understanding the Enigmatic F Element
The halogens, a reactive group of elements, reside in the Group 17 (VIIA) of the periodic table. Among them shines the nonmetallic element fluorine (F), a captivating subject of chemical study.
Characteristics and Reactivity of Halogens
Halogens are known for their electronegativity and strong oxidizing properties. They readily form covalent bonds with other elements, eagerly accepting electrons to complete their outermost electron shells. This intense reactivity makes halogens indispensable in various industrial and everyday applications.
Group 17 (VIIA) Placement
Fluorine’s position in Group 17 indicates it possesses seven valence electrons, the electrons in its outermost shell, which play a pivotal role in chemical bonding. This electronic configuration influences fluorine’s properties and reactivity.
Nonmetallic Properties
Fluorine, like other nonmetals, exhibits a negative electron affinity, attracting electrons from other atoms. It lacks the luster and malleability of metals and forms molecular compounds rather than ionic ones. These characteristics make fluorine an essential component in a wide range of chemical reactions and applications.
Unveiling the Secrets of Valence Electrons: Unraveling the Chemistry of Fluorine (F)
In the realm of chemistry, valence electrons play a pivotal role in determining the reactivity and bonding behavior of elements. Embark on a journey to discover the fascinating world of valence electrons and delve into the intriguing case of the element F (Fluorine).
Determining Valence Electrons in F
The group number of an element provides a valuable clue to the number of valence electrons it possesses. Fluorine resides in Group 17 (VIIA), indicating the presence of seven valence electrons.
Delving deeper into the electron configuration of F (1s²2s²2p⁵), we find confirmation of its valence electron count. The five electrons occupying the 2p orbital constitute the valence electrons of F.
Understanding the Electron Configuration of F and Valence Electron Count
An electron configuration provides a detailed blueprint of the distribution of electrons within an atom. For F, the 1s²2s² represents the core electrons, while the 2p⁵ electrons are the valence electrons. These valence electrons are the ones that participate in chemical reactions, determining the element’s reactivity and bonding properties.
Real-Life Applications of Valence Electrons in F
Understanding valence electrons is crucial for comprehending the chemical bonding and reactivity of F. The seven valence electrons of F allow it to readily form covalent bonds by sharing its electrons with other atoms.
This valence electron configuration contributes to the high reactivity of F, making it an essential component of many compounds and reactions. For instance, the formation of hydrofluoric acid (HF), a corrosive acid used in glass etching and semiconductor manufacturing, involves the sharing of valence electrons between F and H atoms.
By unraveling the secrets of valence electrons in F, we gain invaluable insights into the chemical principles that govern the behavior of this fascinating element, enabling us to harness its properties for various applications.
Electron Configuration of Fluorine (F)
Fluorine, a highly reactive element, is the first member of the halogens and belongs to Group 17 (VIIA) of the periodic table. Its unique chemical properties are directly influenced by the number and arrangement of its electrons, specifically its valence electrons.
Valence Electrons: The Gateway to Chemical Bonding
Valence electrons reside in the outermost energy level of an atom and play a crucial role in determining its chemical behavior. In the case of fluorine, its seven valence electrons govern its reactivity and ability to form chemical bonds. This number is directly related to fluorine’s position in Group 17, where elements have seven electrons in their outermost energy level.
Electron Configuration: A Map to Electronic Distribution
The electron configuration of fluorine, 1s²2s²2p⁵, provides a detailed blueprint of its electron distribution. The 2p orbital contains five electrons, which constitute fluorine’s valence electrons. This configuration underscores the importance of these outermost electrons in shaping fluorine’s chemical interactions.
Applications of Valence Electrons in Fluorine’s Chemistry
Fluorine’s valence electrons not only determine its reactivity but also influence its bonding behavior. Fluorine tends to form one covalent bond with another atom by sharing its valence electrons. This behavior is evident in the formation of compounds such as hydrogen fluoride (HF), where fluorine shares one of its valence electrons with hydrogen. The reactivity and bonding properties of fluorine directly stem from its unique valence electron configuration.
Chemical Bonding and Valence Electrons in Fluorine (F)
Fluorine, a highly reactive element, plays a crucial role in various chemical reactions due to its unique valence electrons. In this section, we will delve into the captivating world of fluorine’s bonding behavior and unravel the secrets of its molecular interactions.
Valence electrons are the electrons in the outermost shell of an atom, responsible for chemical bonding. Fluorine belongs to Group 17 (also known as Group VIIA) of the periodic table, indicating that it has seven valence electrons. These electrons reside in its 2p orbital.
In chemical bonding, fluorine tends to share its valence electrons to achieve a stable electron configuration. It typically forms covalent bonds, where it shares its valence electrons with other atoms to create molecular orbitals.
The reactivity and bonding properties of fluorine are directly linked to its valence electron configuration. Fluorine’s high electronegativity (ability to attract electrons) and small size make it an ideal partner for forming strong covalent bonds.
In summary, fluorine’s seven valence electrons and the properties associated with them play a fundamental role in determining its chemical bonding behavior. Understanding these concepts provides a deeper appreciation of the role that valence electrons play in shaping the molecular world around us.
Real-Life Applications of Valence Electrons in Fluorine (F)
Fluorine, a highly reactive element, is found in numerous compounds and plays a significant role in various industries. Its unique properties stem from the behavior of its valence electrons, which are the electrons in its outermost shell that participate in chemical bonding.
Reactivity and Bonding Behavior
Fluorine’s seven valence electrons make it highly electronegative, meaning it has a strong affinity for electrons. This makes it a powerful oxidizing agent, readily reacting with other elements to form compounds. In chemical bonding, fluorine typically forms one covalent bond by sharing its valence electrons.
Compounds and Reactions
Fluorine’s valence electrons are crucial in the formation of various compounds. One prominent example is hydrofluoric acid (HF), used in the production of semiconductors and etching glass. In HF, fluorine’s valence electron interacts with hydrogen’s valence electron, forming a covalent bond.
Fluorine is also essential in fluoropolymers, such as Teflon, which are highly resistant to heat and chemicals. These polymers have strong carbon-fluorine bonds formed by fluorine’s valence electrons sharing with carbon’s valence electrons.
Additionally, fluorine’s reactivity with water results in the formation of hydrofluoride ions (HF) and hydrogen gas (H ). This reaction is utilized in the production of fluorine gas and the synthesis of certain chemicals.
Fluorine’s valence electrons are central to its reactivity and bonding behavior. They enable fluorine to form compounds with a wide range of elements, making it useful in various industries. Understanding the role of valence electrons in fluorine provides a deeper appreciation of its chemical properties and practical applications.