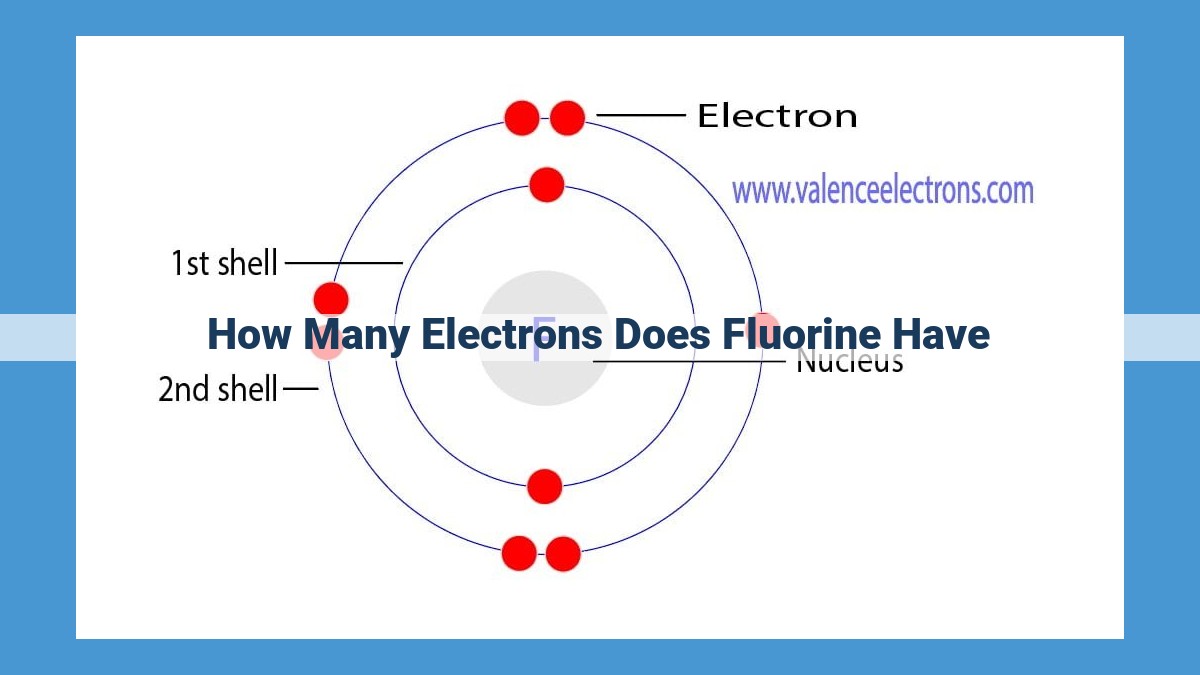
Fluorine, with an atomic number of 9, has 9 electrons. Its electron configuration is 1s²2s²2p⁵, indicating five valence electrons in the outermost p-orbital. These valence electrons play a crucial role in fluorine’s high reactivity as it tends to gain one electron to achieve a stable octet configuration, resulting in the formation of fluoride ions (F⁻).
Atomic Structure: Unraveling the Building Blocks of Matter
At the heart of every atom, a miniature universe unfolds. Protons, nestled within the nucleus, carry a positive charge, while electrons, circling like planets around a star, possess an equal but negative charge. The harmonious dance between protons and electrons dictates the atomic number, which defines an element’s unique identity.
Delving deeper into the atomic core, we encounter neutrons, neutral particles that contribute to an atom’s mass. The mass number represents the combined count of protons and neutrons, providing a glimpse into an atom’s overall hefty nature. The nucleon number, the sum of protons and neutrons, tells us about the building blocks that constitute an atom’s nucleus.
Every atom strives to reach a stable equilibrium, a state of inner harmony. This stability is held together by binding energy, the glue that keeps protons and neutrons cozy within the nucleus. Binding energy ensures that atoms maintain their integrity, preventing them from disintegrating like a house of cards in a gentle breeze.
Atomic and Nuclear Reactions
- Briefly discuss radioactivity and nuclear decay, providing examples.
- Introduce nuclear fission and fusion, highlighting their energy-releasing properties.
Atomic and Nuclear Reactions: The Drama of Matter’s Transformation
The realm of atomic and nuclear reactions is a captivating saga of matter’s transformative powers. At the heart of these processes lies radioactivity, a phenomenon where unstable atoms release energy to achieve a more stable state. Think of it as a cosmic ballet where atoms dance, shedding excess energy like sparkling starbursts.
Nuclear decay occurs when an atom spontaneously breaks down, spewing out particles like alpha particles (helium nuclei) and beta particles (electrons). Take uranium-238, for example; it undergoes a series of alpha and beta decays, eventually transforming into stable lead-206.
Then, there are the powerhouses of the universe: nuclear fission and nuclear fusion. Fission involves splitting heavy nuclei like uranium-235 into lighter elements, releasing enormous energy. It’s the principle behind nuclear power plants and atomic bombs. Fusion, on the other hand, combines light nuclei like hydrogen into heavier elements, also releasing energy. It’s the process that fuels stars and is being harnessing for future energy sources.
These reactions paint a vivid tapestry of matter’s chameleon-like nature, constantly transforming and reshaping the building blocks of our world. They’re not just scientific concepts; they’re the heartbeat of the universe, shaping everything from the stars above to the atoms within our own bodies.
Electrons and Their Properties
- Describe electron configuration and its significance.
- Explain the concepts of valence electrons, electron cloud, and electron density.
- Discuss electron affinity, ionization energy, and electronegativity.
Electrons and Their Enigmatic Properties
In the world of atoms, electrons play a crucial role in shaping their properties and determining their behavior. Let’s delve into their multifaceted nature, starting with electron configuration.
Each element has a unique arrangement of electrons in its energy levels, known as its electron configuration. This configuration governs the element’s chemical reactivity and many other properties. For instance, elements with a full outermost energy level, like noble gases, are highly stable and less likely to react.
Valence electrons are the electrons in the outermost energy level, and they play a significant role in chemical bonding. These electrons determine how an element interacts with other atoms and forms molecules. The number of valence electrons directly impacts an element’s chemical properties.
Electrons don’t exist as tiny particles orbiting the nucleus like planets around a star. Instead, they occupy a three-dimensional electron cloud, a region of space where the probability of finding an electron is highest. The electron cloud is not static; it constantly fluctuates, giving electrons a wave-like character.
Electron density, a measure of the number of electrons present in a specific region of space, helps visualize the electron cloud. Higher electron density indicates a greater probability of finding electrons in that region.
Electrons also possess certain energy levels. Electron affinity is the energy released when an electron is added to an atom, while ionization energy is the energy required to remove an electron from an atom. These properties influence an element’s stability, reactivity, and ability to form ions.
Finally, electronegativity measures an atom’s ability to attract electrons towards itself in a chemical bond. It reflects the atom’s pull on electrons and influences the polarity of chemical bonds. Understanding these properties is essential for comprehending the behavior of atoms and molecules.
Fluorine’s Electron Count: Unveiling the Secrets of a Reactive Element
In the realm of chemistry, electrons play a pivotal role in shaping the behavior and reactivity of elements. Among them, fluorine stands out as a particularly intriguing element, thanks to its unique electron configuration. Let’s delve into the fascinating world of fluorine’s electron count and discover its profound implications in chemical reactions.
Atomic Number and Electron Count
Every element is characterized by its unique atomic number, which represents the number of protons in its nucleus. Fluorine, with an atomic number of 9, has 9 protons. This, in turn, dictates the number of electrons orbiting the nucleus to maintain electrical neutrality, resulting in 9 electrons for fluorine.
Electron Configuration and Distribution
The electron configuration of an element describes the arrangement of its electrons in energy levels around the nucleus. Fluorine’s electron configuration is 1s²2s²2p⁵. This notation indicates that two electrons occupy the first energy level (1s), two electrons are in the second energy level (2s), and the remaining five electrons are in the third energy level (2p).
Valence Electrons and Chemical Reactivity
The electrons in the outermost energy level, known as valence electrons, play a crucial role in determining an element’s chemical reactivity. In fluorine’s case, it has seven valence electrons, occupying the 2p orbital. These valence electrons are loosely bound to the nucleus, making fluorine highly reactive.
Fluorine’s high chemical reactivity stems from its стремление к to attain a stable octet of valence electrons—eight electrons in its outermost energy level. This стремление к drives fluorine to form bonds with other elements, either by gaining or sharing electrons to achieve a stable electron configuration.
Fluorine’s electron count of nine, with seven valence electrons in the 2p orbital, is a defining characteristic that shapes its chemical behavior. This electron configuration makes fluorine highly reactive, leading to its involvement in numerous chemical reactions and forming compounds with various elements. Understanding fluorine’s electron count is essential for comprehending its role in chemistry and its applications in various fields.