Gallium, a captivating Group 13 element, possesses unique characteristics that stem from its valence electrons. With three valence electrons, gallium is poised for versatile chemical bonding, contributing to its semiconducting properties and diverse applications. Its electron configuration reveals the key to understanding its reactivity and the fascinating world of chemistry that revolves around valence electrons.
Valence Electrons: The Building Blocks of Chemical Relationships
In the bustling realm of chemistry, understanding the language of atoms is paramount. One crucial concept that forms the very foundation of this intricate language is valence electrons. These energetic electrons, residing in the outermost shell of an atom, hold the key to unlocking the mysteries of chemical bonding and reactivity.
The number of valence electrons an atom possesses is governed by its position on the periodic table. As we traverse from left to right across a period, the number of valence electrons steadily increases, giving rise to a predictable pattern that shapes the chemical properties of elements. This underlying arrangement influences their ability to form bonds with other elements, determining their chemical behavior and the potential reactions they may undergo.
Beyond their pivotal role in chemical bonding, valence electrons also exert a profound impact on the physical properties of elements. Their presence or absence can dictate whether an element exhibits characteristics of a metal, nonmetal, or metalloid. For instance, metals possess a high number of valence electrons, making them excellent conductors of heat and electricity, while nonmetals have a low number, resulting in their insulating properties. Metalloids, like the enigmatic gallium, occupy a fascinating middle ground, displaying a captivating blend of both metallic and nonmetallic traits.
By delving deeper into the concept of valence electrons, we lay the groundwork for a comprehensive understanding of chemistry, paving the way for unraveling the secrets of atomic interactions and the intricate dance of chemical reactions.
Gallium: A Unique Group 13 Element
In the realm of chemistry, the element gallium stands out as an enigmatic figure. Its metalloid nature bridges the gap between metals and non-metals, showcasing a captivating blend of properties that have captivated scientists and engineers alike.
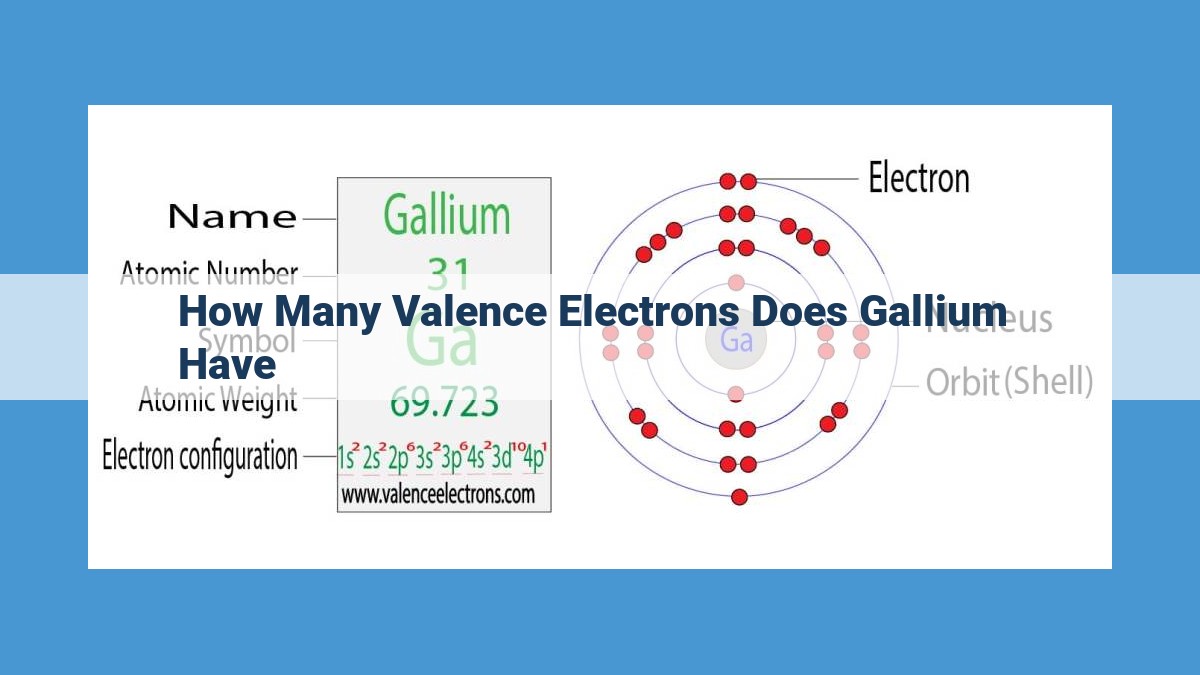
As a member of Group 13 on the periodic table, gallium shares kinship with other elements such as aluminum and indium. However, it distinguishes itself with its unique electron configuration that bestows upon it remarkable characteristics. Gallium’s atomic structure features a total of 31 electrons, with its outermost shell accommodating three valence electrons. This seemingly simple arrangement holds the key to understanding gallium’s exceptional behavior.
Gallium’s valency of three endows it with a peculiar ability to form covalent bonds with other atoms. These strong and directional bonds play a pivotal role in determining gallium’s physical and chemical properties. Interestingly, gallium’s low melting point and high boiling point further underscore its metalloid nature, defying the boundaries that typically separate metals from non-metals.
Perhaps the most captivating aspect of gallium lies in its semiconducting properties. This exceptional quality enables gallium to conduct electricity under specific conditions, making it an indispensable component in various electronic devices. Transistors, integrated circuits, and light-emitting diodes all rely on the unique properties of gallium to function.
Gallium’s Valence Electrons: Unlocking Its Potential
As we delve into the realm of chemistry, the concept of valence electrons plays a crucial role in understanding the behavior and properties of elements. In this article, we will explore the fascinating world of gallium, a unique Group 13 element, and unravel the secrets of its valence electrons.
Identifying Gallium’s Valence Electrons
Every atom consists of a nucleus surrounded by electrons. Valence electrons are those electrons that occupy the outermost shell of an atom. Gallium, an element with an atomic number of 31, has an electron configuration of [Ar]3d¹⁰4s²4p¹. The superscript numbers indicate the number of electrons in each subshell. Gallium has three valence electrons in its 4p subshell.
Why Three Valence Electrons?
The number of valence electrons is determined by the element’s position in the periodic table. Group 13 elements have three valence electrons because they have three electrons in their outermost energy level. This unique electron configuration gives gallium its distinctive properties and plays a significant role in its chemical behavior.
Gallium’s Semiconducting Nature
Semiconductors are materials that can conduct electricity under certain conditions. Gallium’s three valence electrons allow it to behave as a semiconductor. When an electric field is applied, the valence electrons can move freely between atoms, facilitating the flow of electricity. This property makes gallium an essential component in electronic devices such as transistors and light-emitting diodes (LEDs).
Furthermore, gallium’s semiconducting properties can be tailored by adding impurities, known as doping. By doping gallium with elements like arsenic or phosphorus, its electrical conductivity can be controlled, making it suitable for a wide range of applications.
Unveiling Gallium’s Potential
Gallium’s unique electron configuration and semiconducting properties have unlocked its potential in various fields:
- Electronics: Gallium-based semiconductors are used in high-speed transistors, integrated circuits, and solar cells. Its semiconducting properties enable efficient and reliable electronic devices.
- Medical Imaging: Gallium-67 is a radioisotope used in medical imaging techniques. It is injected into the body and accumulates in areas of inflammation, providing valuable diagnostic information.
- Corrosion Protection: Gallium can be used as an alloying element in aluminum to enhance corrosion resistance. The presence of gallium forms a protective oxide layer that inhibits corrosion.
Gallium’s valence electrons are the key to its unique properties and wide-ranging applications. By understanding the nature of its valence electrons, we can harness gallium’s potential to drive advancements in various fields. From electronics to medical imaging, gallium’s versatility continues to inspire innovation and shape the future of technology.