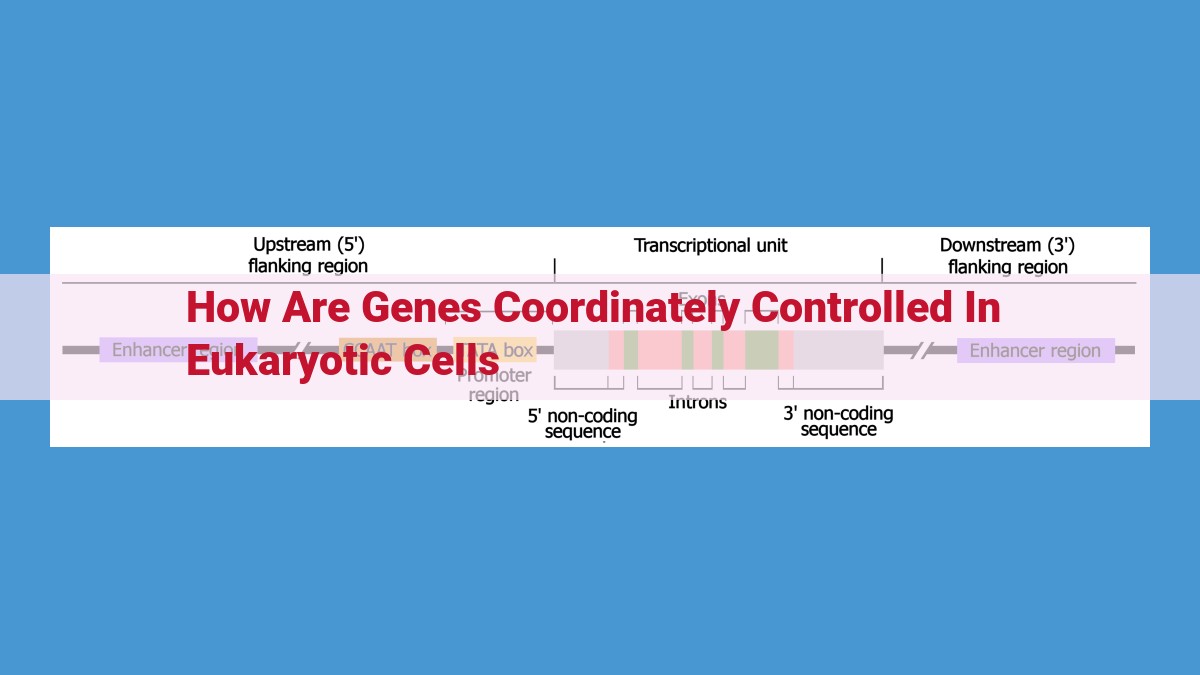
Genes in eukaryotic cells are intricately coordinated by a complex interplay of enhancers, promoters, transcription factors, and chromatin modifiers. Enhancers act as masterminds, interacting with promoters to enable gene expression. Promoters serve as gatekeepers, regulating transcription through interactions with enhancers and regulatory proteins. Transcription factors operate as master regulators, binding to enhancers and promoters to recruit coactivators or corepressors, influencing gene activation or repression. Mediator bridges transcription factors and RNA polymerase II, facilitating transcription initiation. Coactivators enhance transcription by promoting enhancer-promoter interactions and recruiting chromatin remodeling complexes. Corepressors suppress transcription by blocking enhancer-promoter interactions and recruiting chromatin modifiers. Chromatin remodeling complexes alter histone packing, affecting gene accessibility. Histone modifications, known as epigenetic regulators, impact gene expression by altering chromatin structure. Together, these factors orchestrate coordinated gene control, orchestrating cellular processes and influencing cellular differentiation.
Enhancers: The Masterminds of Gene Expression
In the captivating symphony of gene control, enhancers play the role of masterful conductors, orchestrating the harmonious expression of genes. These enigmatic regulatory elements, scattered throughout our chromosomes, possess an unparalleled ability to interact with distant promoters and transcription factors, akin to a celestial choreographer connecting far-apart celestial bodies.
Enhancers serve as “landing pads” for transcription factors, the key regulators that determine whether a gene is turned on or off. Through this interaction, enhancers bridge the gap between distal regulatory elements and the gene’s promoter, the command center for gene transcription.
But the path from enhancer to promoter is not always straightforward. Here’s where chromatin remodeling complexes step in as the backstage crew. These molecular machines remodel the tightly packed chromatin structure, allowing enhancers to communicate with their target promoters. Histone modifications, subtle chemical changes to histones (the spools around which DNA is wound), further influence the accessibility of enhancers. By loosening or tightening the chromatin fibers, these modifications pave the way for transcription factors to access and interact with enhancers, ensuring the seamless flow of genetic information.
Enhancers are not merely static bystanders in gene regulation. They respond dynamically to cellular cues and environmental signals, adapting their activity to meet the ever-changing needs of the cell. This dynamic nature of enhancers underlies the intricate complexity of gene control, orchestrating the precise expression of genes in response to a symphony of internal and external stimuli.
Promoters: Gatekeepers of Gene Activation
In the intricate world of gene expression, promoters stand as pivotal gatekeepers, meticulously regulating when and how genes spring to life. These regulatory regions reside adjacent to genes, acting as docking stations for the molecular machinery that orchestrates transcription, the process of transcribing DNA into RNA.
Promoters function as strategic control points, determining which genes are active and when. They possess specific sequences of nucleotides that serve as binding sites for a cast of regulatory proteins, including transcription factors. These proteins act as messengers, conveying signals from external stimuli or cellular cues to the promoter.
When a transcription factor binds to its cognate sequence within the promoter, it triggers a cascade of events. The promoter undergoes a conformational change, opening up the DNA for access by RNA polymerase II, the enzyme responsible for transcribing genes into RNA. This molecular ballet is further orchestrated by coactivators, which facilitate the binding of RNA polymerase II to the promoter and enhance transcription.
However, the promoter’s accessibility is not static. It is subject to the dynamic interplay of chromatin remodeling complexes and histone modifications. Chromatin remodeling complexes are molecular machines that alter the packaging of DNA around histone proteins, making the promoter either more or less accessible. Histone modifications, such as acetylation and methylation, can further fine-tune promoter accessibility, influencing the ability of transcription factors and RNA polymerase II to bind.
Thus, promoters serve as intricate control points in gene regulation, integrating signals from transcription factors, coactivators, chromatin remodelers, and histone modifiers to orchestrate the precise expression of genes.
Transcription Factors: The Master Regulators of Gene Expression
In the intricate world of gene regulation, there are key players known as transcription factors. These potent molecular switches orchestrate the symphony of gene expression, acting as gatekeepers that determine whether genes are turned on or off.
Transcription factors possess the remarkable ability to recognize specific DNA sequences within enhancers and promoters, regions of our genetic material that control gene transcription. When they bind to these DNA targets, they initiate a cascade of molecular events that either promote or suppress gene activation.
Imagine a transcription factor as a skilled conductor, bringing together a team of coactivators and corepressors. These molecular partners act as supporting players, either facilitating or hindering the binding of the transcription factor to DNA. By interacting with coactivators, transcription factors strengthen their grip on DNA and recruit other proteins that further promote gene transcription. Conversely, corepressors act as silencers, blocking the transcription factor’s interaction with DNA and hindering gene expression.
However, the stage for transcription factor action is not always straightforward. The chromatin, the tightly packed structure of our DNA, can present obstacles to accessibility. Here, another group of molecular architects, known as chromatin remodeling complexes, intervene. These master manipulators alter the chromatin landscape, allowing transcription factors to access their DNA targets.
Finally, another layer of regulation comes into play: histone modifications. These chemical alterations to the histone proteins that package our DNA influence transcription factor activity. Certain modifications act as flags, attracting transcription factors to specific genes, while others serve as roadblocks, hindering their binding and suppressing gene expression.
Thus, transcription factors stand as the conductors of gene regulation, working in concert with a team of supporting players to control the flow of genetic information. By orchestrating a symphony of events, they determine the fate of genes, shaping the cellular landscape and driving the biological functions of our bodies.
Mediator: The Mastermind Behind Transcription Initiation
Imagine a bustling city, where gene expression is the key to life. In this intricate metropolis, transcription factors serve as the city’s architects, designing the buildings (genes) that will shape the city’s future. However, these architects cannot work alone; they need a mediator—a bridge—to connect them to the construction workers (RNA Polymerase II)—Mediator.
Mediator is a protein complex that serves as the intermediary between transcription factors and RNA Polymerase II, the molecular machine that transcribes DNA into RNA. It acts as a platform, bringing together the architects (transcription factors) and the construction workers (RNA Polymerase II), allowing them to communicate and collaborate effectively.
Just as a skilled mediator can facilitate harmony between parties, Mediator helps to align the actions of transcription factors, ensuring that they work together to orchestrate the symphony of gene expression. Mediator binds to specific sites on DNA, known as Mediator binding sites, and serves as an anchor for transcription factors. It then uses its assembly factor function, recruiting coactivators (helpers for transcription) and RNA Polymerase II to the transcription complex.
By facilitating the assembly of the transcription complex, Mediator ensures that the right genes are expressed at the right time and in the right amount, shaping the city’s destiny and ensuring its optimal functioning.
Coactivators: The Unsung Heroes of Gene Expression
In the intricate dance of gene regulation, coactivators emerge as the indispensable allies, facilitating the symphony of gene expression. These enigmatic proteins possess the remarkable ability to amplify the voices of transcription factors, enabling them to exert their influence on the stage of gene regulation.
Coactivators forge strong bonds with transcription factors, acting as their loyal lieutenants. This partnership empowers transcription factors to bind more effectively to enhancers, the command centers that dictate the initiation of gene expression. Moreover, coactivators possess a unique talent: they can bridge the gap between enhancers and promoters, allowing the directives from afar to reach the genes themselves.
But the brilliance of coactivators extends far beyond mere matchmaking. They marshal the forces of chromatin remodeling complexes and histone modifiers, the architects of chromatin structure. By loosening the grip of tightly packed chromatin, these coactivators create a more welcoming environment for transcription factors, allowing them to access the genes they are destined to regulate.
In essence, coactivators serve as the facilitators, ensuring that transcription factors can execute their mission: activating genes and orchestrating the cellular symphony. Without these unsung heroes, the melody of gene expression would falter, and the harmony of cellular life would be lost.
Corepressors: Inhibitors of Transcription
- Explain the role of corepressors in suppressing gene expression.
- Describe their binding to transcription factors and their ability to block enhancer-promoter interactions.
- Discuss the involvement of corepressors in recruiting chromatin remodeling complexes and histone modifiers.
Corepressors: The Unsung Heroes of Gene Silencing
In the intricate tapestry of gene regulation, where DNA weaves a symphony of cellular activity, there exists a hidden protagonist – the corepressor. These molecular gatekeepers quietly work to suppress gene expression, playing a crucial role in the delicate balance of cellular processes.
Unveiling the Suppressive Nature of Corepressors
Corepressors, as their name suggests, are the masterminds behind gene silencing. They attach themselves to transcription factors, the conductors of gene activation, effectively disrupting their ability to usher in the RNA polymerase II orchestra. This disruption blocks the crucial connection between enhancers and promoters, preventing the initiation of gene transcription.
Binding to Transcription Factors: A Silent Embrace
Corepressors exert their inhibitory power by latching onto transcription factors. This binding is not merely a passive interaction – it’s a strategic maneuver that sabotages the transcription factor’s ability to assemble the full transcriptional machinery. With the key players neutralized, gene expression grinds to a halt.
Silencing Enhancer-Promoter Interactions
Enhancers are the beacons that guide RNA polymerase II to the correct gene location, while promoters are the entry point for transcription. Corepressors, with their potent silencing capabilities, interfere with these interactions. They cloak the enhancers, making them invisible to RNA polymerase II, and they barricade the promoters, preventing any unauthorized access.
Recruiting Repressive Allies: Chromatin Remodelers and Histone Modifiers
Corepressors excel at multitasking, and their repertoire extends beyond direct transcription factor inhibition. They conspire with chromatin remodeling complexes and histone modifiers to create an environment that is hostile to gene expression. Chromatin remodelers rearrange the DNA packaging, making it inaccessible to transcription factors, while histone modifiers chemically tweak the chromatin structure to further reinforce the silencing effect.
In the bustling city of the cell, corepressors are the silent guardians of gene silencing. They act as a formidable barrier against inappropriate gene activation, ensuring that the cellular symphony remains harmonious. By suppressing unwanted melodies, corepressors maintain cellular balance and prevent the cacophony of unregulated gene expression. Their unsung role is essential for the smooth operation of our biological machinery.
Chromatin Remodeling Complexes: Architects of Gene Expression
Introduction:
Chromatin, the intricate packaging of DNA within eukaryotic cells, plays a crucial role in regulating gene expression. At the heart of this regulation are chromatin remodeling complexes, molecular machines that dynamically alter the structure of chromatin to facilitate gene access.
Function of Chromatin Remodeling Complexes:
These complexes, like skilled architects, manipulate the tightly packed histone proteins around DNA, allowing transcription factors and RNA polymerase to reach their target genes. By altering the accessibility of DNA, they act as gatekeepers, controlling the flow of genetic information.
Enhancer-Promoter Communication:
Chromatin remodeling complexes play a particularly crucial role in bridging the gap between enhancers and promoters. Enhancers, distant DNA regions that regulate gene expression, can be located far from the genes they control. Chromatin remodeling complexes extend their reach, creating chromatin loops that bring enhancers into proximity with promoters, enabling them to communicate and coordinate gene activation.
Facilitating Transcription Factor Binding:
Moreover, chromatin remodeling complexes ensure that transcription factors, the key regulators of gene expression, can bind to their target DNA sequences. By unwinding tightly packed chromatin, they expose the binding sites and create a more accessible environment for transcription factors to interact with DNA and initiate transcription.
Conclusion:
Chromatin remodeling complexes are essential for the precise orchestration of gene expression in eukaryotes. Their ability to remodel chromatin structure, facilitating communication between enhancers and promoters and enabling transcription factor binding, makes them pivotal players in controlling the flow of genetic information and shaping cellular identity.
Histone Modifications: The Epigenetic Regulators
In the intricate world of gene regulation, histone modifications play a crucial role as epigenetic regulators. These modifications alter the structure of histone proteins, the building blocks of chromatin, which packages DNA within the nucleus. By influencing the accessibility of DNA to transcription factors, histone modifications orchestrate gene expression and orchestrate cellular differentiation.
Histones undergo various chemical alterations, including acetylation, methylation, phosphorylation, and ubiquitination. These modifications can loosen or tighten chromatin structure, determining which genes are “on” or “off.” Acetylation, for instance, relaxes chromatin, making DNA more accessible and promoting gene expression. In contrast, methylation can condense chromatin, hindering transcription factor binding and gene activation.
The impact of histone modifications extends beyond individual genes. They can influence enhancer-promoter interactions, which are essential for coordinating gene expression across long distances. Enhancers are DNA regions that act as distant switches, controlling the expression of target genes. Histone modifications that facilitate enhancer-promoter communication enable precise gene regulation in response to external cues or developmental signals.
Histone modifications also play a critical role in cellular differentiation. As cells specialize in different functions, their gene expression profiles must adapt accordingly. Histone modifications create epigenetic landscapes that determine which genes are accessible for transcription in each cell type. This epigenetic information can be stably maintained throughout cell division, ensuring the preservation of differentiated cell identities.
Therefore, histone modifications are indispensable epigenetic regulators that empower cells to control gene expression with precision. They orchestrate the coordination of gene activity, facilitating cellular differentiation and adapting to changing environments. By understanding the intricacies of histone modifications, we can unravel the mysteries of gene regulation and gain insights into the fundamental processes that govern life.