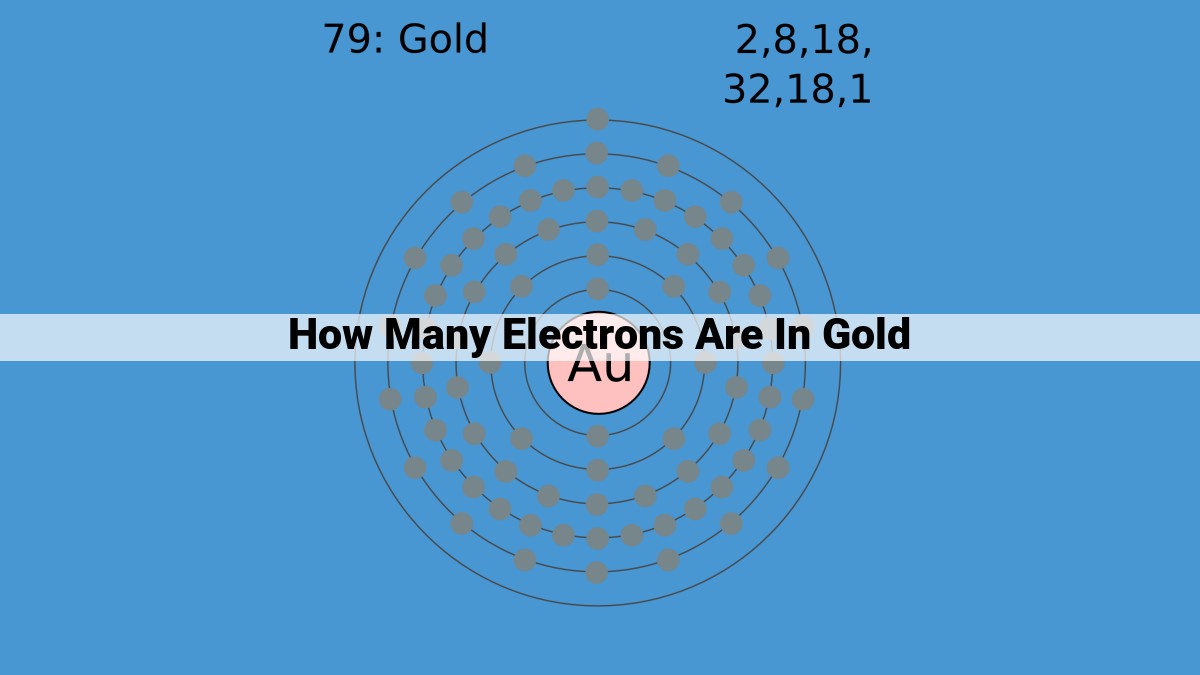
Gold, with an atomic number of 79, contains 79 electrons. These electrons are organized in an electron configuration of 2, 8, 18, 32, 18, 1, indicating the number of electrons in each energy level. This electron configuration gives gold unique chemical properties and contributes to its distinctive golden color, malleability, and corrosion resistance.
Atomic Number: The Identity of Gold
In the realm of elements, each one bears a unique fingerprint known as its atomic number, which is akin to an element’s passport. This numerical identity plays a pivotal role in distinguishing one element from another, much like a fingerprint distinguishes one person from another.
Gold, the lustrous metal prized for its beauty and resilience, boasts a distinctive atomic number of 79. This unique number places gold smackdab in the 11th group and 6th period of the periodic table, a roadmap of all known elements. The atomic number of an element is not merely a random number; it unveils the very essence of the element, determining its chemical behavior and shaping its interactions with the world around it.
Electronic Configuration: Unveiling the Inner Workings of Gold
The allure of gold, beyond its shimmering beauty, lies in its unique atomic structure. At the heart of this structure lies its electron configuration, a blueprint that orchestrates the arrangement of electrons within its atomic orbitals.
Just like the blueprint of a grand mansion, the electron configuration of gold reveals the distribution of its 79 electrons across distinct energy levels, known as electron shells. These shells, like concentric layers, surround the nucleus, housing electrons in an organized hierarchy of energy.
At the innermost core resides the first electron shell, a compact abode for just two electrons. The second shell, slightly larger, comfortably accommodates eight electrons. Moving outward, the third shell expands its capacity to 18 electrons, while the fourth shell dwarfs them all, sheltering 32 electrons.
Finally, the outermost electron shell, like a protective mantle, holds the remaining 18 electrons. However, one of these electrons breaks free from the shell’s embrace, becoming a solitary wanderer known as a valence electron. This lone electron plays a pivotal role in gold’s chemical behavior.
The arrangement of electrons within these shells, known as the electron configuration of gold, is expressed as 2, 8, 18, 32, 18, 1. It not only defines gold’s identity but also governs its chemical bonding and reactivity.
The valence electron that ventures out of the outermost shell eagerly seeks companionship, forming the foundation of chemical bonds. This electron’s quest for interaction determines how gold interacts with other elements, shaping its diverse applications and the remarkable properties that have captivated humankind for centuries.
The Role of Protons in the Gold Atom’s Nucleus
The core of every gold atom is its nucleus, a tiny, dense region where the atom’s protons reside. These positively charged particles are the fundamental building blocks of an element’s identity.
Gold, with its atomic number 79, boasts an impressive 79 protons in its nucleus. This unique number differentiates gold from all other elements, giving it its distinct characteristics and properties.
The number of protons in an atom’s nucleus also has a profound impact on its isotopic composition. Isotopes are variations of the same element that differ in the number of neutrons. While gold’s atomic number remains constant at 79, it can have varying numbers of neutrons, resulting in different isotopes.
For instance, gold-197, the most common isotope, contains 79 protons and 118 neutrons. Its mass number, the sum of protons and neutrons, is 197. Other isotopes, such as gold-195 and gold-196, have different numbers of neutrons and, consequently, different mass numbers.
Understanding the number of protons in gold’s nucleus is crucial for comprehending the element’s isotopic composition, its behavior in chemical reactions, and its various applications in science, industry, and art.
Number of Neutrons: Neutral Builders of the Nucleus
Neutrons, the unassuming partners in the atomic nucleus, play a crucial role in the story of gold’s atomic structure. These neutral particles possess neither an electrical charge nor an independent will. Yet, their presence has a profound impact on the mass and diversity of gold’s atomic forms.
Within the nucleus, neutrons stand side by side with protons, the positively charged particles that define an element’s atomic number. The balance between protons and neutrons determines the stability and isotopic composition of an element. Gold, with its atomic number of 79, boasts an equal number of protons and 118 neutrons in its most common isotope, gold-197.
Neutrons, like silent partners, contribute significantly to the atomic mass of gold. Each neutron adds a unit to the mass number, which is the sum of protons and neutrons in the nucleus. Gold-197, with its 79 protons and 118 neutrons, has a mass number of 197. This distinction is crucial for understanding the various isotopic forms of gold and their unique properties.
Isotopes, like fraternal twins, share the same atomic number but differ in their neutron count. Gold, with its versatile nucleus, exhibits multiple isotopes, each with varying numbers of neutrons. Gold-195, for instance, has two fewer neutrons than gold-197, while gold-196 has one neutron less. These isotopes, though chemically similar, have distinct physical properties and find diverse applications.
Neutrons, the neutral builders of the nucleus, are not mere bystanders in gold’s atomic symphony. They contribute to the element’s mass, diversity, and the tapestry of its unique properties. Unveiling their significance is like uncovering a secret code that unlocks the mysteries of gold’s atomic structure and its profound impact on the world we experience.
Mass Number: Counting the Essence of the Gold Atom’s Nucleus
In the realm of atomic structure, the mass number stands as a pivotal concept, unveiling the total number of particles that reside within an atom’s nucleus. This enigmatic number holds the key to understanding the interplay between protons, the positively charged particles, and neutrons, their uncharged counterparts, that reside at the heart of every atom.
The Sum of Nuclear Particles
The mass number, represented by the symbol A, is simply the sum of the number of protons and neutrons within the nucleus. Protons, with their inherent positive charge, dictate an element’s identity and its position on the periodic table. Neutrons, on the other hand, lack any electrical charge but contribute significantly to the atom’s mass.
Gold-197: An Illustrative Example
Take, for instance, the isotope known as gold-197. Its mass number, as the name suggests, is 197. This implies that the nucleus of gold-197 houses a total of 197 particles. Since gold’s atomic number is 79, which signifies the number of protons, we can deduce that gold-197 contains 197 – 79 = 118 neutrons.
Implications for Atomic Structure
The mass number plays a crucial role in determining various aspects of an atom’s structure and behavior. For example, it influences the atomic mass, which is the weighted average of the masses of all isotopes, and the nuclear stability. Isotopes with a high neutron-to-proton ratio tend to be more stable, as neutrons provide a stabilizing force against the electrostatic repulsion between protons.
Understanding the concept of mass number is essential for unraveling the intricacies of atomic structure. By grasping the sum of protons and neutrons within an atom’s nucleus, we gain insights into the unique properties and behavior of elements like gold. This understanding serves as a cornerstone for comprehending the world of chemistry, materials science, and beyond.
Isotopes of Gold: Unraveling the Variations
In the realm of chemistry, isotopes play a captivating role in shaping the diversity of elements. They are atomic siblings that share the same elemental identity but possess distinctive weights due to variations in their neutron count. Gold, the shimmering metal known for its allure and versatility, is no exception to this captivating dance of atoms.
Gold’s atomic nucleus, the heart of the atom, harbors a mass number that represents the combined number of protons and neutrons. While all gold atoms share the unwavering atomic number of 79, denoting their unique position in the periodic table, they exhibit individuality in their neutron count. This subtle difference gives rise to intriguing variations within the gold family, known as isotopes.
Among the known isotopes of gold, gold-197 stands out as the most prevalent, accounting for over 99% of natural gold. Its mass number of 197 reflects the presence of 79 protons and 118 neutrons, imparting a characteristic weight to this isotope.
But the gold family extends beyond gold-197. Gold-195 and gold-196 are additional isotopes that, while less abundant, contribute to the element’s diverse isotopic landscape. Gold-195, with 116 neutrons, boasts a mass number of 195, while gold-196, with 117 neutrons, has a mass number of 196.
These isotopic variations not only influence the mass of gold atoms but also contribute to the element’s nuclear stability. Gold-197, with its balanced neutron-to-proton ratio, is the most stable isotope, explaining its dominance in nature. Gold-195 and gold-196, with slightly different neutron counts, exhibit varying degrees of stability and find applications in specialized fields, such as nuclear medicine and geological studies.
Unveiling the intricate tapestry of gold’s isotopes provides a deeper appreciation for the complexities of the atomic world. It showcases the subtle yet profound impacts of neutron count on an element’s identity, stability, and applications, making gold a captivating subject for both scientific exploration and practical endeavors.