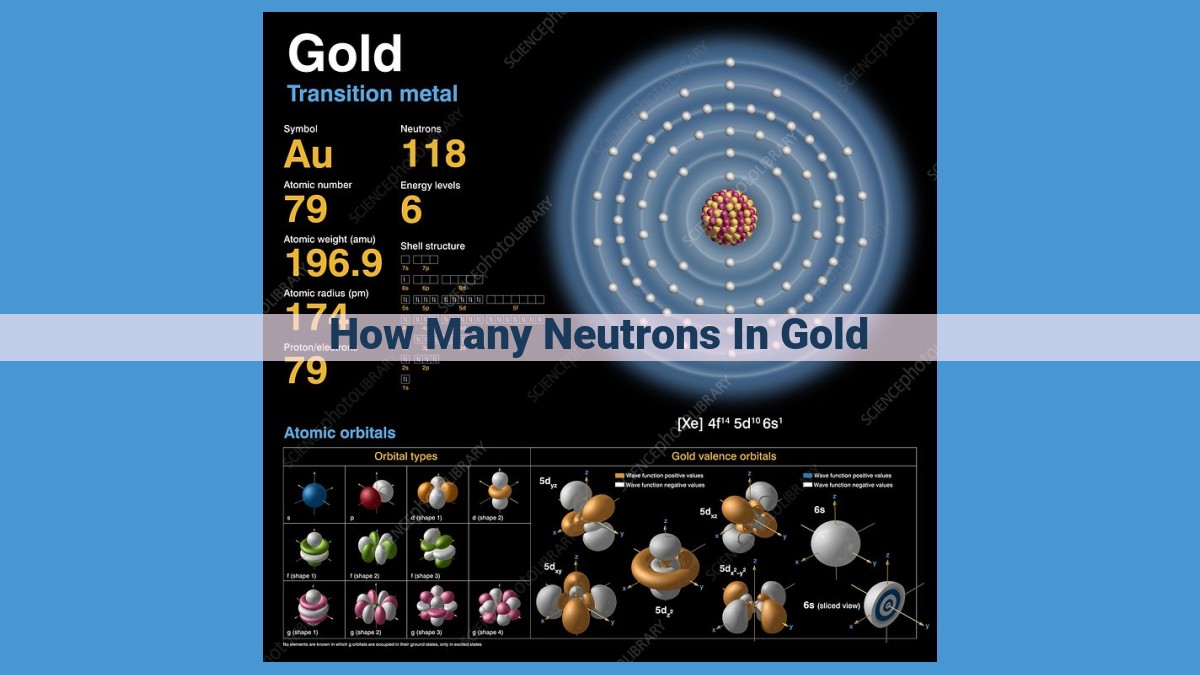
The atomic number of gold (79) identifies the element. The mass number of gold, typically 197, represents the combined number of protons and neutrons. Since gold has 79 protons, it has 118 neutrons in its most abundant isotope, 197Au. The number of electrons in an electrically neutral atom is equal to the number of protons, so gold has 79 electrons.
Atomic Number of Gold
- Explain the concept of atomic number and how it identifies each element.
- State the atomic number of gold (79).
Understanding the Atomic Number of Gold
Gold, a precious and captivating metal, holds a unique place in the world of elements. Its distinctive characteristics stem from its fundamental atomic structure, and one crucial aspect of this is its atomic number.
The Enigma of Atomic Numbers
Each element in the periodic table possesses a unique atomic number, akin to an identity card that sets it apart from others. This number represents the number of protons, the positively charged particles found in the heart of an atom, in its nucleus. Protons determine an element’s fundamental properties, its chemical behavior, and its place within the periodic table.
Gold’s Signature: Atomic Number 79
In the case of gold, its atomic number shines forth as 79. This number signifies that every single gold atom contains within its nucleus a steadfast troupe of 79 protons. These protons orchestrate the chemical reactions and interactions that define gold’s unique nature. Understanding the atomic number of gold, therefore, grants us a glimpse into its fundamental essence.
Unraveling the Mass of Gold: A Journey into Atomic Architecture
Gold, with its captivating luster and timeless allure, has intrigued humankind for centuries. Beyond its aesthetic appeal, gold possesses an intriguing atomic structure that reveals its composition and unique properties. Let’s embark on an exploration of the mass number of gold and delve into the fascinating world of its isotopic variations.
The Significance of Mass Number
The mass number of an element represents the total number of protons and neutrons found in its nucleus. This value plays a crucial role in determining the atom’s atomic weight and isotopic identity. Isotopes are variations of the same element that share the same atomic number but differ in their neutron count.
Gold’s Mass Number and Isotopic Diversity
The mass number of gold is 197. This indicates that the nucleus of a gold atom contains 79 protons (as determined by its atomic number) and 118 neutrons. However, gold exhibits isotopic diversity, meaning there are multiple isotopes of gold with varying neutron counts.
The most common isotope of gold is 197Au. It accounts for over 99.9% of all gold found on Earth. Its stable nucleus consists of 79 protons and 118 neutrons, giving it a mass number of 197.
Isotopic Variations: A Tale of Neutron Fluctuations
While 197Au is the most abundant isotope, other isotopes of gold exist. These isotopes have different neutron counts, leading to variations in their mass numbers. For instance, 195Au and 198Au are less common isotopes with mass numbers of 195 and 198, respectively.
The existence of isotopes underscores the dynamic nature of atomic structure. Elements can exhibit variations in their neutron counts without altering their chemical properties. This isotopic diversity contributes to the unique characteristics and applications of each element in various scientific fields.
Delving into the Heart of Gold: Unveiling Its Atomic Architecture
In the realm of elements, gold stands out with its captivating allure and remarkable properties. Its atomic structure, a intricate symphony of subatomic particles, holds the key to understanding its unique characteristics. Among these particles, protons play a pivotal role in defining the element’s identity.
Protons: The Cornerstone of Atomic Identity
Within the nucleus, the heart of an atom, protons reside as the building blocks of its positive charge. Each element possesses a unique number of protons, acting as a fingerprint that distinguishes it from all others. This inherent characteristic, known as atomic number, serves as the defining feature of an element.
Gold’s Unwavering Atomic Number
In the case of gold, its atomic number of 79 firmly establishes its position in the periodic table. This number represents the immutable quantity of protons that make up every gold atom. These protons, with their unwavering positive charge, not only influence the atom’s electrical properties but also dictate its chemical behavior.
The Invisible Force Shaping Element Identity
The significance of protons extends beyond their mere presence. They exert a profound influence on the atom’s overall charge. In an electrically neutral atom, the number of protons is precisely balanced by the number of electrons, negatively charged particles that orbit the nucleus. This delicate equilibrium between protons and electrons ensures the atom’s stability and its ability to interact with other atoms.
Unveiling the Number of Protons in Gold
To fully unravel the atomic structure of gold, we must delve into the concept of isotopes. Isotopes are variations of an element that share the same atomic number but differ in their number of neutrons. While all gold atoms contain 79 protons, the number of neutrons can vary, giving rise to different isotopes.
The most prevalent isotope of gold, accounting for approximately 100% of naturally occurring gold, is 197Au. This isotope boasts 79 protons and 118 neutrons, giving it a mass number of 197.
By understanding the number of protons in gold and its ramifications, we gain a deeper appreciation for the intricacies of atomic structure. It is this fundamental knowledge that unlocks the secrets of gold’s remarkable properties, shaping its unique place in our world.
The Enigmatic Electrons of Gold: Unveiling the Secrets of Atomic Structure
In the realm of chemistry, gold stands as a precious metal renowned for its captivating luster and enduring value. Beyond its captivating appearance, gold possesses a fascinating atomic structure that holds the key to understanding its unique properties. Among the fundamental aspects of this structure is the number of electrons that orbit its nucleus, a concept that we will delve into today.
In the vast expanse of the atomic universe, each element is assigned a unique atomic number, which represents the number of protons residing within its nucleus. For gold, this atomic number is 79, indicating the presence of 79 positively charged protons. To maintain electrical neutrality, an atom must possess an equal number of negatively charged electrons. Therefore, gold atoms harbor a total of 79 electrons.
These electrons are not mere spectators within the atomic structure; they play a pivotal role in shaping gold’s properties and behavior. They are arranged in specific energy levels, or orbitals, around the nucleus, with each orbital accommodating a certain number of electrons. The electron configuration of gold is as follows:
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1
This configuration reveals that gold has a single electron in its outermost energy level, known as the valence shell. This valence electron is responsible for gold’s high reactivity and its ability to form bonds with other atoms. It is also responsible for gold’s characteristic yellow color.
The number of electrons in an atom plays a crucial role in determining its chemical properties. As such, gold’s 79 electrons endow it with unique reactivity and affinity for other elements. This explains gold’s wide range of applications, from jewelry and coinage to electronics and medicine.
Unveiling Gold’s Inner Secrets: Exploring its Atomic Makeup
Delving into the Heart of Gold: Number of Neutrons
Gold, an element revered for its lustrous beauty and economic significance, holds fascinating secrets within its atomic structure. Neutrons, uncharged particles residing in the nucleus alongside protons, play a crucial role in defining the identity of this precious metal.
The atomic mass number represents the total number of protons and neutrons in an atom’s nucleus. For gold, its mass number is 197, indicating the presence of both 79 protons and 118 neutrons in its most common isotope, denoted as ¹⁹⁷Au.
Isotopes are variations of an element that share the same atomic number but differ in neutron count. Gold exhibits several isotopes, each with a distinct number of neutrons. The most stable and abundant isotope is ¹⁹⁷Au, comprising over 99% of naturally occurring gold.
The number of neutrons in an atomic nucleus influences its stability and radioactive properties. Isotopes with an uneven number of neutrons are often radioactive and decay over time, while isotopes with an even number of neutrons tend to be more stable.