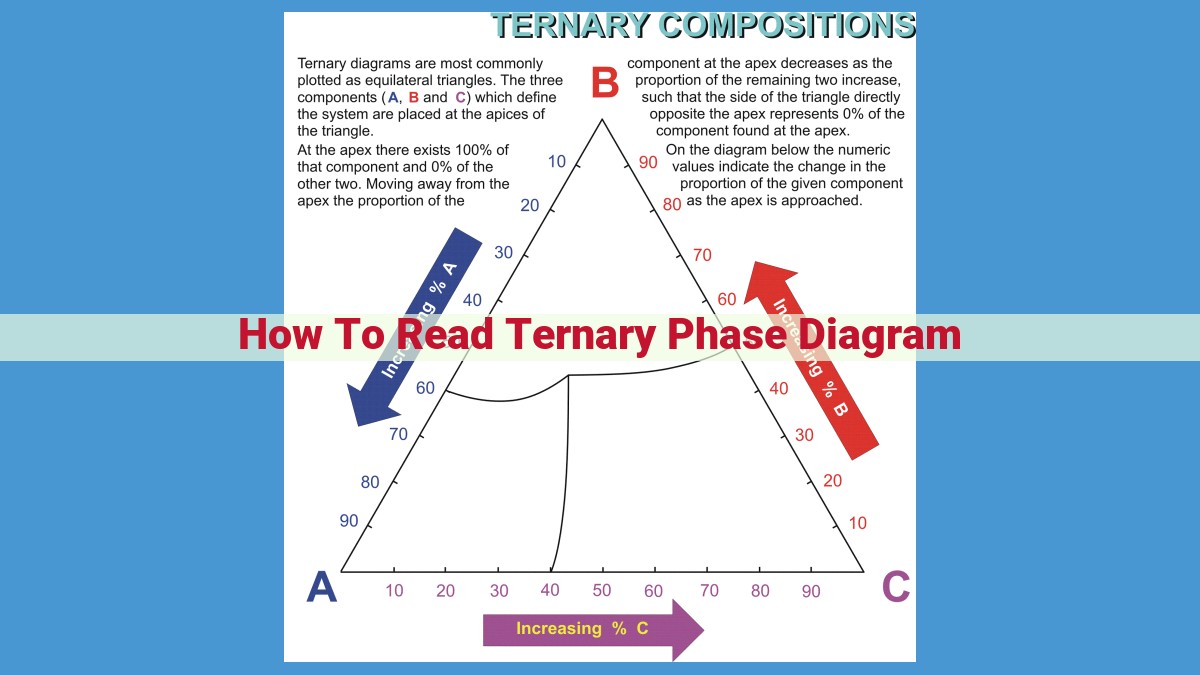
To comprehend ternary phase diagrams, begin by understanding the phase triangle, where each vertex represents a pure component and the interior points represent combinations. Identify the different phases (solid, liquid, gas) and their characteristics. Observe special points such as eutectic and peritectic points where phase transformations occur. Analyze congruent and incongruent transformations to understand how compositions change during phase transitions. By mastering these concepts, you can effectively utilize ternary phase diagrams to predict phase behaviors and optimize material properties in diverse applications.
Ternary Phase Diagrams: Unraveling the Secrets of Material Behavior
In the realm of materials science and chemical engineering, understanding the behavior of complex multi-component systems is crucial. Enter ternary phase diagrams—a powerful tool for visualizing and predicting phase transformations and equilibrium states in materials containing three components.
Phase diagrams provide a graphical representation of the phase (solid, liquid, or gas) of a system under varying conditions of temperature and composition. For ternary systems, these diagrams take the form of equilateral triangles, with each vertex representing one component and the sides representing binary mixtures of those components.
Ternary phase diagrams hold immense value in a wide range of applications. They enable scientists and engineers to:
- Predict and optimize material properties, such as strength, hardness, and conductivity.
- Control phase transformations during processing and fabrication.
- Design materials with tailored microstructures and functionalities.
By understanding the principles behind ternary phase diagrams, researchers and practitioners can unlock the secrets of material behavior and harness their knowledge to create innovative materials for cutting-edge technologies.
Understanding Phase Relationships with Gibbs Phase Rule
In the realm of materials science and chemical engineering, understanding the behavior of materials under varying conditions is crucial. Ternary phase diagrams play a pivotal role in this regard, providing a graphical representation of the phases present in a system composed of three components.
The Gibbs Phase Rule is a fundamental principle that governs the number of phases that can coexist in a system at equilibrium. It states that the maximum number of phases is given by:
P = C - F + 2
where:
- P is the number of phases
- C is the number of components
- F is the number of degrees of freedom (temperature, pressure, concentration)
Introducing Binary Phase Diagrams
Before delving into ternary systems, it’s essential to understand binary phase diagrams. These diagrams represent the phase equilibria of a system composed of two components, such as a metal and a non-metal. The diagram plots the temperature and composition at which different phases form.
Binary phase diagrams typically consist of lines (phase boundaries) that separate regions where different phases exist. These phases can include solid, liquid, and gas states. By understanding the relationships between temperature, composition, and phase, scientists and engineers can predict the behavior of binary systems in various applications.
Ternary Systems: Unveiling the Complexity of Multi-Component Phase Diagrams
In the realm of materials science and chemical engineering, ternary phase diagrams serve as invaluable tools for comprehending the intricate relationships between multiple components within a system. These diagrams provide a visual representation of the various phases that coexist under specific conditions of temperature, pressure, and composition.
The Phase Triangle: A Gateway to Ternary Compositions
To delve into the world of ternary systems, we introduce the phase triangle—a versatile graphical device that allows us to visualize compositions involving three components. Each vertex of the triangle represents a pure component, while points within the triangle denote mixtures of two or all three components. The relative distances between points indicate the proportions of each component in the mixture.
Components: The Building Blocks of Ternary Systems
The three components that comprise a ternary system are of paramount importance. Each component possesses unique properties and characteristics that contribute to the overall behavior of the system. By understanding the individual characteristics of the components, we can begin to unravel the complex interactions that occur within the system.
For example, consider a ternary system composed of water, alcohol, and salt. Water and alcohol are miscible (i.e., they readily dissolve in each other), while salt is immiscible in both water and alcohol. This fundamental difference in solubility has a profound impact on the phase diagram of the system.
Phase Types in Ternary Phase Diagrams
Phase diagrams provide a valuable tool for understanding the complex behavior of multicomponent systems. In ternary systems, involving three components, phase transformations and phase equilibria play a significant role in determining the properties and applications of materials.
Solid Phase
Solids are characterized by a rigid and ordered atomic arrangement. In a ternary phase diagram, solid phases are represented by triangles, with the corners representing the pure components and the compositions of the solid phase lying within the triangle. The properties of solid phases vary widely depending on their crystal structure, such as melting point, hardness, and electrical conductivity.
Liquid Phase
Liquids are fluid and have a disordered atomic arrangement. On a ternary phase diagram, liquid phases are typically represented by irregular shapes. They are typically characterized by a lower viscosity and higher mobility compared to solids.
Gas Phase
Gases are highly diffusive and have a very low density. They are not typically represented on ternary phase diagrams, as they are assumed to be in a separate state of equilibrium. Gases can affect the behavior of solid and liquid phases by influencing their partial pressures and interfacial phenomena.
Each phase has unique characteristics and properties that influence the overall behavior of the ternary system. Understanding the phase types is crucial for materials scientists and chemical engineers to design and optimize materials for specific applications. By carefully analyzing the phase diagram and considering the properties of each phase, researchers can tailor materials with desired microstructures and performance characteristics.
Special Points on Phase Diagrams
- Eutectic point: coexistence of two solids and a liquid
- Peritectic point: solid-liquid equilibrium with phase transformation
Special Points on Ternary Phase Diagrams: A Tale of Coexistence and Transformation
Ternary phase diagrams are essential tools in materials science and chemical engineering, providing valuable insights into the complex interactions between three different components. Among the various features on these diagrams, two prominent points stand out: the eutectic and peritectic points.
The Magic of Eutectic Points: Coexistence in Harmony
Imagine a trio of friends, each with their own unique preferences. However, there’s a magical point where they can all get along perfectly: the eutectic point. In a ternary phase diagram, the eutectic point represents the temperature and composition at which two solids and a liquid can coexist in equilibrium.
Just like our trio of friends, the solids and liquid have their own personalities and properties. However, at the eutectic point, they find a harmonious balance. The liquid and one of the solids solidify simultaneously, forming a fine-grained mixture with unique characteristics.
Peritectic Points: A Puzzle with Pieces Changing Form
Now, let’s introduce another twist to our story. Instead of friends who get along perfectly, we have a duo of friends who aren’t quite so harmonious: the peritectic point. Here, a solid and a liquid exist in equilibrium, but with a catch. The solid undergoes a phase transformation, evolving into a different solid.
Imagine two friends who start out as best pals but gradually drift apart and become slightly different. At the peritectic point, the original solid gradually transforms into its new incarnation, creating a unique coexistence of two solids and a liquid. Just like our friends’ evolving relationship, the phase transformation at the peritectic point leads to a new equilibrium state.
understanding these special points on ternary phase diagrams helps scientists and engineers predict and modify the properties of materials. By manipulating the temperature and composition, they can create materials with tailored characteristics for a wide range of applications, from high-performance alloys to advanced composite materials.
Phase Transformations in Ternary Systems
In the realm of metallurgy and materials science, ternary phase diagrams play a pivotal role in unraveling the complex interactions between multiple components. These diagrams depict the equilibrium phase relationships within a system, guiding researchers and engineers in materials design and processing. Phase transformations, the heart of these diagrams, hold significant importance in understanding how materials evolve under varying conditions.
Congruent Transformations
One type of phase transformation is congruent, where the parent and daughter phases share the same composition. Imagine a solid parent phase that undergoes a transformation to form a liquid daughter phase. As the temperature rises, the parent phase dissolves into the liquid, maintaining a constant composition throughout the process. This harmonious transition results in a smooth curve on the phase diagram, resembling a melting point line.
Incongruent Transformations
In contrast, incongruent transformations involve a parent phase with a composition distinct from the daughter phase. Here, the parent phase reacts with the liquid to form a new solid phase. As temperature increases, the parent phase partially melts, forming a liquid and a new solid phase. This reaction continues until the parent phase is entirely consumed, giving rise to a distinctive kink in the phase diagram.
For instance, in a ternary system composed of iron, carbon, and chromium, the austenite phase (γ) can undergo an incongruent transformation to form the ferrite phase (α) and a liquid phase. This transformation plays a crucial role in the heat treatment of steel, influencing its strength and hardness properties.
Understanding the intricacies of phase transformations is essential for manipulating the properties of materials. By carefully controlling temperature and composition, scientists and engineers can tailor materials for specific applications. Ternary phase diagrams serve as invaluable tools in this pursuit, providing a roadmap for the intricate dance of phases within complex material systems.