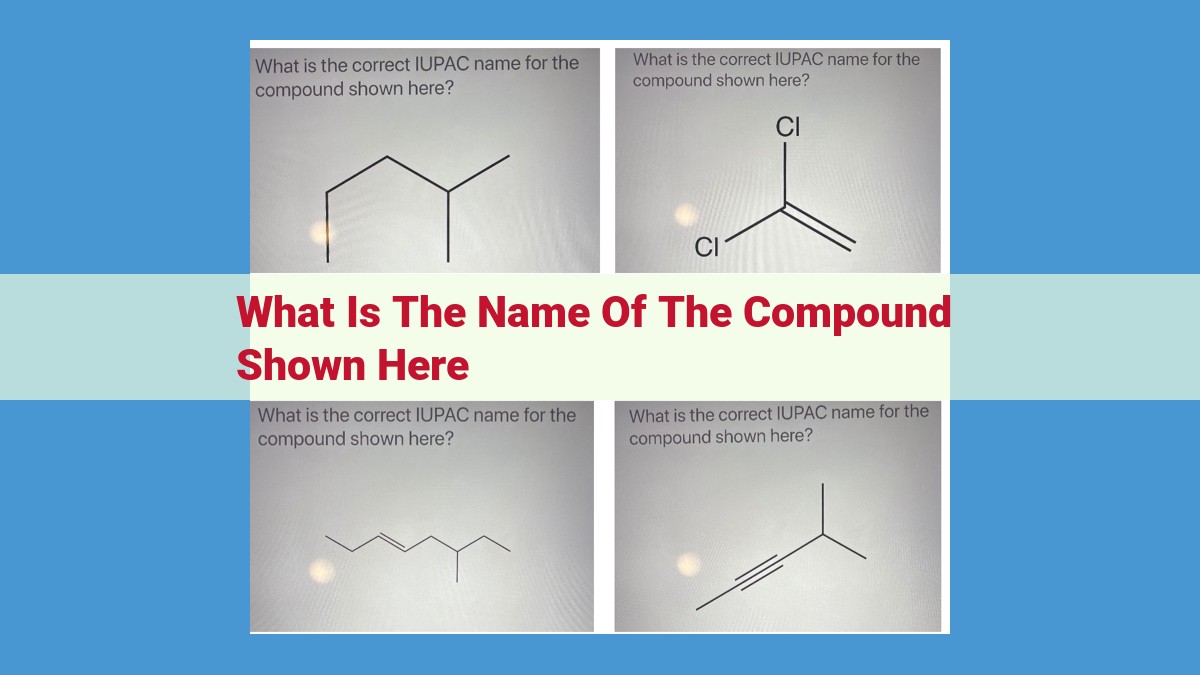
The name of the compound is not provided in the given text. However, the text discusses key concepts related to naming compounds, including the molecular formula, structural formula, and IUPAC guidelines. The molecular formula represents the elemental composition of the compound, while the structural formula depicts the spatial arrangement of atoms and bonds. The IUPAC guidelines provide a systematic way to name organic compounds based on their functional groups and structural features.
Understanding the Essence of Molecular Formulas: The Building Blocks of Compounds
Unraveling the Molecular Formula: The Identity Key
In the realm of chemistry, compounds reign supreme, and understanding their composition is crucial. The molecular formula serves as the cornerstone of this endeavor, providing a window into the elemental makeup of these intricate substances. A molecular formula, represented as an array of chemical symbols and numeric subscripts, reveals the exact number and types of atoms that constitute a compound.
Decoding the Molecular Formula: Beyond Elemental Composition
The molecular formula goes beyond mere elemental identification. It unlocks the door to related concepts that illuminate the nature of compounds. The chemical composition indicates the proportions of elements within a compound, while the empirical formula offers a simplified representation of the simplest whole-number ratio of atoms present. Together, these concepts enhance our grasp of compound composition.
The Structural Formula: A Visual Symphony of Atoms
Moving beyond the elemental blueprint, the structural formula unveils the spatial choreography of atoms within a molecule. Unlike the molecular formula, which focuses solely on composition, the structural formula captures the intricate dance of atoms, revealing the precise arrangement of bonds and functional groups. This visual representation empowers chemists to decipher the molecular architecture, predicting reactivity and properties.
Interwoven Concepts: Bridging the Molecular Formula and Structural Formula
The molecular formula and structural formula form an intertwined tapestry of information. The molecular formula provides the raw materials, while the structural formula breathes life into the molecular edifice, revealing the spatial relationships and connectivity of atoms. Understanding the interplay of these concepts is paramount for unraveling the complexities of the chemical world.
Unveiling the Secrets of Compounds: A Journey of Discovery
The molecular formula stands as a gateway to unraveling the enigmatic world of compounds. It provides the elemental blueprint, paving the way for deeper insights into chemical composition and structure. Armed with this knowledge, chemists embark on a journey of discovery, unravelling the mysteries that lie within the molecular realm.
Structural Formula: Visualizing Molecules
In the realm of chemistry, understanding the intricate dance of atoms and their bonding arrangements is crucial. This is where the structural formula steps into the spotlight, providing a powerful tool to visualize the spatial orientation of atoms within a molecule.
Unlike the molecular formula that simply reveals the elemental makeup of a compound, the structural formula delves deeper, offering a pictorial representation of the molecule’s architecture. It depicts the precise arrangement of each atom, along with the bonds connecting them.
To unravel the significance of the structural formula, let’s venture into the world of related concepts:
-
Lewis Structure: A simplified version of the structural formula, highlighting the distribution of electrons involved in chemical bonding.
-
Ball-and-Stick Model: A three-dimensional representation of a molecule, where atoms are represented as balls and bonds as sticks.
These tools play a vital role in comprehending the behavior and properties of molecules. By understanding the spatial relationships between atoms, we can gain insights into:
-
Molecular geometry and shape
-
Reactivity and bonding patterns
-
Physical and chemical properties
Moreover, the structural formula serves as a bridge between the molecular formula and the macroscopic properties of a substance. It provides the essential blueprint for understanding the behavior of materials at the atomic level, paving the way for advancements in fields such as drug design, material science, and nanotechnology.
Naming Compounds: Unveiling the Identity of Molecules
In the realm of chemistry, substances dance and combine, forming intricate structures that possess unique identities. To unravel these identities, we turn to the art of naming compounds, a crucial step in understanding their composition and properties.
Types of Names: A Rich Tapestry of Identifiers
Throughout history, compounds have been bestowed with a myriad of names, each serving a specific purpose. Chemical names, also known as systematic names, follow a set of standardized rules to describe the exact structure of the compound. Common names, on the other hand, are colloquial terms derived from the compound’s properties or origins, such as sugar (sucrose) or salt (sodium chloride). Last but not least, IUPAC names are the official nomenclature system established by the International Union of Pure and Applied Chemistry, providing a globally recognized way to name organic compounds.
Unveiling the Structure with IUPAC Nomenclature
For organic compounds, IUPAC nomenclature stands as the gold standard, guiding chemists in assigning unambiguous names. This system relies on identifying the parent chain—the longest continuous chain of carbon atoms—and any functional groups attached to it. Functional groups are specific arrangements of atoms that impart characteristic properties to the compound.
Once these elements are identified, the name is assembled following a specific set of rules. The prefix of the name indicates the number of carbon atoms in the parent chain, while the suffix reflects the functional group present. For example, the compound with the parent chain containing three carbon atoms and an alcohol functional group is named propanol.
The Power of Precision
Precise naming is not merely an academic exercise. It allows scientists to communicate about compounds with clarity and accuracy, ensuring that everyone is referring to the same substance. This precision is vital in research, manufacturing, and countless other applications where understanding the identity of a compound is essential.
In the vast world of chemistry, naming compounds is a crucial skill, empowering scientists to decipher the identity of substances, unlock their secrets, and harness their potential for the betterment of society.