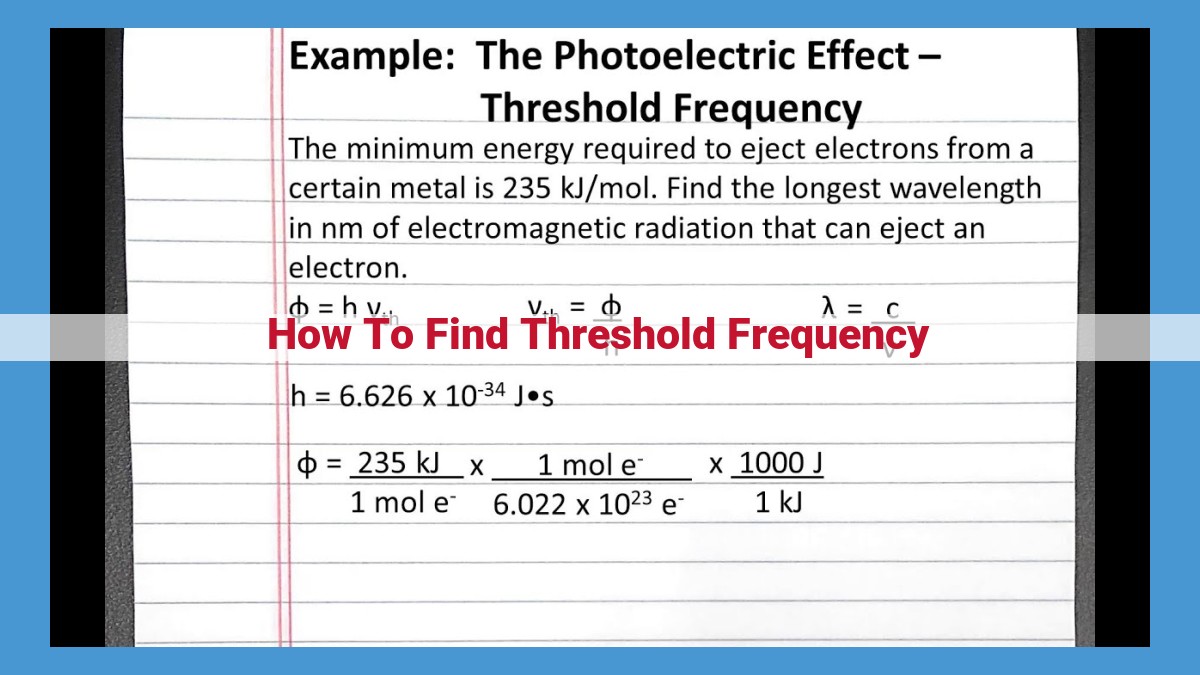
To find the threshold frequency, start by determining the work function of the material in question. This is the energy required to remove an electron from the material. The kinetic energy of the emitted electrons is directly proportional to the frequency of the incident light minus the threshold frequency. By plotting the kinetic energy of the emitted electrons as a function of the frequency of the incident light, the threshold frequency can be found as the x-intercept of the graph.
Delving into the Photoelectric Effect: A Tale of Light and Electrons
In the realm of science, discoveries often arise from the depths of observation and experimentation. One such discovery that transformed our understanding of light and matter was the photoelectric effect, a phenomenon that unveiled the fundamental nature of light and its interaction with electrons.
The photoelectric effect, first observed by physicist Heinrich Hertz in the late 19th century, occurs when light strikes a metal surface, causing electrons to be emitted. This seemingly simple observation held profound implications for our understanding of the universe and paved the way for advancements in electronics and technology.
Understanding the Photoelectric Effect
To delve into the details of the photoelectric effect, we must first grasp the fundamental concepts that underlie it. Light, in its essence, is an electromagnetic wave composed of photons, tiny packets of energy. On the other hand, electrons are the basic building blocks of matter, negatively charged particles that reside within atoms.
When light strikes a metal surface, the photons that constitute the light interact with the metal’s electrons. If the photon has sufficient energy, it can transfer that energy to an electron, liberating it from the metal’s grip. This liberated electron, now possessing kinetic energy, is emitted from the surface.
The key to understanding the photoelectric effect lies in the energy exchange between photons and electrons. The energy of a photon, determined by its frequency, must exceed a certain threshold to initiate the photoelectric effect. This threshold, known as the work function, is unique to each metal and represents the minimum energy required to extract an electron from its clutches.
Implications and Applications
The photoelectric effect has revolutionized our understanding of the interaction between light and matter and has had far-reaching implications in various fields of science and technology. It laid the foundation for the development of photocells, devices that convert light energy into electrical energy, and photomultipliers, instruments that amplify faint light signals.
The photoelectric effect also finds applications in spectroscopy, a technique used to identify and characterize materials based on their interaction with light.
The photoelectric effect stands as a testament to the power of scientific inquiry and the profound insights it can reveal. By unraveling the nature of light and electrons, this discovery opened up new avenues of research and innovation, shaping our understanding of the universe and enabling groundbreaking technological advancements. Its legacy continues to inspire scientists and engineers to explore the hidden realms of nature and harness its power for the betterment of humankind.
Key Concepts of the Photoelectric Effect
Delving into the realm of quantum physics, we encounter a fascinating phenomenon known as the photoelectric effect. This effect, discovered by Albert Einstein, dramatically challenged classical physics and served as a cornerstone in the development of modern physics. To grasp this intricate concept, let’s unravel its key principles.
Light as a Wave-Particle Duality:
At the core of the photoelectric effect lies the wave-particle duality of light. While often thought of as a wave, light also exhibits particle-like behavior, behaving as discrete packets of energy known as photons. Each photon possesses a well-defined energy, proportional to the frequency of the light.
Electrons: Fundamental Particles of Matter:
Electrons are subatomic particles that orbit the nucleus of an atom. They carry a negative charge and possess wave-like properties. In the context of the photoelectric effect, electrons are liberated from the surface of a material when struck by light.
Energy and the Photoelectric Effect:
Energy plays a pivotal role in the photoelectric effect. When a photon strikes an electron, it can transfer its energy to the electron. This energy transfer can liberate the electron from its bound state within the material, imparting kinetic energy to the freed electron.
Einstein’s Revolutionary Contribution:
Einstein’s groundbreaking explanation of the photoelectric effect revolutionized physics. He proposed that the maximum kinetic energy of emitted electrons is directly proportional to the frequency of the incident light. This relationship, known as Einstein’s photoelectric equation, challenged classical physics, which predicted a continuous increase in electron energy with increasing light intensity.
Mathematical Relationships in the Photoelectric Effect
To understand the intricate workings of the photoelectric effect, we delve into the fundamental concepts of energy and its exchange between light and matter.
The Work Function: A Gatekeeper for Electron Emission
The work function is a material-specific property that represents the minimum energy required for an electron to overcome its electrostatic attraction and be liberated from its atomic bondage. In the photoelectric effect, when a photon strikes the surface of a material, its energy can be transferred to an electron. If the photon carries sufficient energy to overcome the work function, the electron breaks free, becoming a photoelectron.
Kinetic Energy: A Dance with Light Frequency
The kinetic energy of the photoelectrons emitted depends on the frequency of the incident light. As light frequency increases, the energy of the individual photons also increases. This energy transfer directly translates into higher kinetic energy for the emitted photoelectrons. This relationship is linear and can be represented mathematically.
Planck’s Constant: A Quantum-Sized Messenger
Enter Planck’s constant, a fundamental physical constant that connects the world of light and matter. It defines the energy of a photon as a function of its frequency. In the photoelectric effect, Planck’s constant acts as a bridge, linking the energy of the incident photon to the energy of the emitted photoelectron.
Threshold Frequency: Unlocking the Gateway to the Photoelectric Effect
The Quantum Barrier
In the realm of light and matter, a fascinating phenomenon known as the photoelectric effect occurs. It’s a dance between photons, the particles of light, and electrons, the fundamental building blocks of atoms. However, for this dance to happen, a certain threshold must be crossed, a threshold frequency.
The Threshold: A Gatekeeper of Electron Excitation
The threshold frequency is a critical value. It represents the minimum frequency of light needed for the photoelectric effect to occur. Below this frequency, no matter how intense the light, no electrons will be liberated from their atomic homes.
The Dance of Light, Electrons, and Energy
When light of a frequency above the threshold strikes a material, the photons carry a specific amount of energy, known as photon energy. This energy corresponds to the frequency of the light. If this energy is higher than the work function of the material, the energy needed to free an electron, a magical dance begins.
The Mathematical Equation: A Guiding Formula
The relationship between the threshold frequency, the work function, and the kinetic energy of the liberated electrons is expressed by the following equation:
hv = Φ + KE
where:
- h is Planck’s constant
- v is the frequency of light
- Φ is the work function
- KE is the kinetic energy of emitted electrons
Experimental Methods: Unveiling the Threshold
Determining the threshold frequency experimentally is a crucial step in understanding its behavior. One method involves using a light source of varying frequencies and observing the onset of the photoelectric effect. By finding the frequency at which the effect begins, the threshold frequency can be pinpointed.
Practical Prowess: Threshold Frequency in Action
The threshold frequency finds practical applications in various technologies, including:
- Photocells: Devices that convert light into electrical current
- Photomultipliers: Instruments that amplify faint light signals
- Medical Imaging: Equipment that utilizes photoelectric detectors to capture images
The concept of threshold frequency is a cornerstone in the study of the photoelectric effect. It provides a deeper understanding of the nature of light and matter and has paved the way for advancements in fields like optoelectronics and medical imaging. This threshold, once crossed, has unlocked a world of possibilities, bridging the gap between photons and electrons in a mesmerizing dance of quantum mechanics.
Practical Applications
- Discuss applications of threshold frequency in photocells, photomultipliers, and other technologies.
Practical Applications of Threshold Frequency
The threshold frequency, a crucial concept in the photoelectric effect, finds invaluable applications in a wide range of optoelectronic technologies. Its discovery opened doors to innovative devices that harness the interaction of light with matter, transforming industries and our daily lives.
Photocells: Detecting Light with Threshold Sensitivity
Photocells, also known as photoconductors, are devices that convert light energy into electrical energy. They rely on the threshold frequency to detect light. When light of a frequency below the threshold falls on the photocell, no electrons are emitted. However, when light exceeds the threshold frequency, electrons are emitted, their number and kinetic energy proportional to the light intensity. This property makes photocells ideal for light detection and measurement.
Photomultipliers: Amplifying Tiny Light Signals
Photomultipliers are sensitive detectors that amplify extremely weak light signals. They utilize the photoelectric effect in multiple stages. When a photon strikes the first photocathode, electrons are emitted due to the threshold frequency effect. These electrons then strike a series of secondary cathodes, each causing the emission of more electrons. The cumulative effect results in a significant amplification of the initial light signal, making photomultipliers essential for detecting and studying low-light phenomena.
Other Applications: From Particle Detection to Night Vision
The threshold frequency principle extends to a variety of other applications. In particle physics, scintillation detectors use the photoelectric effect to convert high-energy particles into light, making particle detection possible. Night vision devices employ photomultipliers to amplify faint starlight, allowing users to see in low-light conditions. Solar cells, which convert sunlight into electricity, also rely on the threshold frequency to generate electrical current.
The concept of threshold frequency, a fundamental aspect of the photoelectric effect, has revolutionized optoelectronics. From the sensitive detection of light in photocells to the amplification of tiny light signals in photomultipliers, threshold frequency plays a pivotal role in enabling a wide range of groundbreaking technologies. Its applications continue to drive advancements in particle detection, night vision, energy harvesting, and beyond.