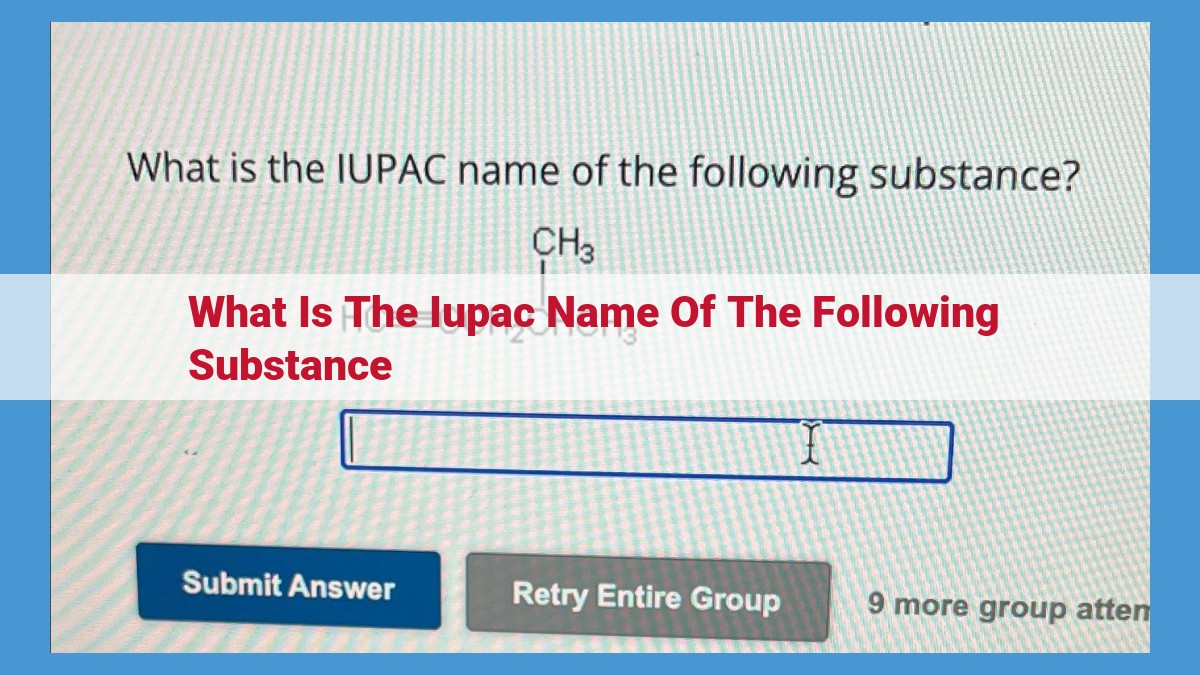
IUPAC nomenclature, a standardized system for naming organic compounds, allows for accurate communication in chemistry. It involves identifying the parent chain, prefixes and suffixes to describe functional groups, and prefixes to represent alkyl groups. The prefixes indicate group number and location, while suffixes denote unsaturation (double or triple bonds) with “-ene” and “-yne,” respectively. Branching complexity is also addressed, describing the location and identity of alkyl groups or functional groups extending from the parent chain.
Decoding the Language of Chemistry: IUPAC Nomenclature
- Introduce IUPAC nomenclature as the standardized system for naming organic compounds, its importance, and why it’s essential for accurate communication in chemistry.
Decoding the Language of Chemistry: Unraveling the Secrets of IUPAC Nomenclature
In the vast lexicon of chemistry, precision is paramount. Accurate naming of compounds ensures crystal-clear communication and understanding among scientists worldwide. Enter IUPAC nomenclature, the standardized system that unlocks the mysteries behind organic compounds, paving the way for groundbreaking discoveries and scientific advancements.
IUPAC nomenclature is the Rosetta Stone of chemistry, enabling us to decipher the structural intricacies of molecules with remarkable clarity. It empowers us to identify compounds, predict their properties, and visualize their molecular blueprints. By adhering to this systematic approach, we align our chemical conversations, fostering collaboration and progress.
Navigating the labyrinthine world of organic compounds can be daunting, but IUPAC nomenclature serves as our guiding star. It offers a structured framework for naming these molecules, ensuring that each compound conveys its identity unambiguously. This precise language eliminates ambiguity and enables scientists to discuss chemical structures with ease and confidence, like deciphering a symphony of molecular notes.
Unveiling the Secrets of Functional Groups: The Building Blocks of Molecular Reactivity
In the realm of chemistry, understanding the language of molecules is crucial for unraveling their behavior and properties. At the heart of this molecular dialogue lies the concept of functional groups, atomic arrangements that govern chemical reactivity like skilled choreographers.
What are Functional Groups?
Functional groups are specific combinations of atoms that impart unique chemical identities to molecules. They are the key players in determining how molecules interact with each other, dictating their solubility, boiling points, and countless other physical and chemical characteristics.
A Glimpse into Common Functional Groups
- Alkyl Groups: Carbon-rich chains, they form the backbone of many organic molecules, acting as the connective tissue that links different functional groups.
- Double Bonds: Represented as “-ene” in IUPAC nomenclature, double bonds consist of two carbon atoms joined by a pair of double bonds. These unsaturated regions introduce new reactivities, making molecules more susceptible to chemical reactions.
- Triple Bonds: Similar to double bonds but with three bonds between two carbon atoms, triple bonds, denoted as “-yne,” further enhance reactivity and confer unique properties.
The Significance of Functional Groups
Functional groups are the cornerstones of molecular interactions. Their presence influences everything from the chemical reactions molecules undergo to their solubility and biological activity. By deciphering the language of functional groups, chemists gain an unparalleled ability to predict and control the behavior of molecules, paving the way for countless scientific advancements.
Alkyl Groups: The Molecular Building Blocks
In the realm of chemistry, where molecules dance in intricate patterns, alkyl groups stand out as the foundational units of organic chemistry. These hydrocarbon chains, devoid of any functional groups, form the backbone of countless compounds and play a crucial role in determining their properties and reactivity.
To delve into the world of alkyl groups, let’s explore their naming conventions. Just as we use prefixes and suffixes in our language to convey specific meanings, alkyl groups follow a set of rules to describe their structure. Their names are derived from the number of carbon atoms in the chain, with prefixes like methyl, ethyl, and propyl indicating the presence of one, two, or three carbon atoms, respectively.
As we add more carbons to the chain, suffixes come into play. Compounds with n carbon atoms are named using the suffix *”-ane” (e.g., pentane for five carbons). This systematic approach ensures that each alkyl group has a unique name that accurately reflects its structure.
But the story of alkyl groups doesn’t end there. Prefixes and suffixes also help us describe the branching patterns within these chains. When alkyl groups extend from the main chain, we use prefixes like iso- and sec- to indicate whether they’re attached to the second or third carbon atom.
These naming conventions may seem complex at first, but they serve as a powerful tool for chemists to communicate precisely about the molecular structures they study. By understanding the language of alkyl groups, we gain a deeper appreciation for the intricate world of organic chemistry.
Finding the Foundation: Parent Chain in Naming
In the world of chemistry, unraveling the complexities of organic compounds can be akin to deciphering a secret code. But fear not, for IUPAC nomenclature, the standardized system for naming these molecules, provides a roadmap to understanding their structure. The parent chain, akin to the foundation of a house, plays a pivotal role in this naming system.
The parent chain is the backbone of an organic compound, the longest continuous chain of carbon atoms that forms the base for its name. It’s like the central pillar of a building, providing stability and serving as the anchor to which other functional groups and branches are attached.
To determine the parent chain, you must first identify the functional groups present – these are specific arrangements of atoms that give compounds their characteristic properties. Functional groups are like the building blocks of organic molecules, each with its own unique set of rules and implications.
Once you’ve identified the functional groups, you can begin to assemble the parent chain. The goal is to find the longest chain of carbons that encompasses all the functional groups. This chain will form the base name of the compound, with prefixes and suffixes added to account for the presence of functional groups and branching.
In a way, naming an organic compound using IUPAC nomenclature is like building a house. The parent chain is the foundation, providing the overall structure and stability. The functional groups are the bricks and mortar, adding specific characteristics and properties. And the prefixes and suffixes are the paint and decorations, giving the house its unique identity. So, when faced with the seemingly daunting task of naming an organic compound, remember the parent chain – it’s the foundation upon which the rest of the naming system is built.
Unveiling Functional Groups with Suffixes: The Gateway to Chemical Reactivity
Unlocking the Secrets of Functional Groups
In the realm of chemistry, functional groups stand out as the atomic architects that govern the behavior of molecules. These unique arrangements of elements dictate a compound’s chemical properties and reactivity.
Suffixes: A Rosetta Stone for Functional Groups
When deciphering the language of organic chemistry, suffixes hold the key to identifying functional groups. Like a molecular Rosetta Stone, they reveal the presence of specific double or triple bonds, providing invaluable clues about a compound’s chemical nature.
“-ene”: A Double Bond Tale
The suffix “-ene” serves as a beacon, signaling the existence of a double bond within the molecule. This bond arises from the sharing of four electrons between two carbon atoms, creating a region of unsaturation. Compounds containing double bonds, known as alkenes, exhibit distinct chemical properties due to their unique electronic structure.
“-yne”: A Triple Bond Adventure
For even greater unsaturation, we turn to the suffix “-yne.” It proclaims the presence of a triple bond, where six electrons are shared between two carbon atoms. This highly reactive bond endows alkynes with a rich array of chemical activities and diverse applications.
Impact on Chemical Properties
Functional groups play a pivotal role in shaping a compound’s chemical reactivity. Double and triple bonds introduce sites of unsaturation, making the molecules more prone to undergo chemical reactions. These reactive bonds can participate in a variety of processes, such as addition, elimination, and substitution, giving rise to a vast spectrum of chemical transformations.
Navigating the Chemical Landscape
Understanding the suffixes associated with functional groups empowers scientists to navigate the complex landscape of organic chemistry. By decoding these molecular cues, researchers gain insights into the structure, properties, and reactivity of compounds, unlocking the secrets that drive chemical transformations and underpin the development of countless technologies.
Prefixes: Delving into Alkyl Groups
- Explain the role of prefixes like “methyl” and “ethyl” in describing alkyl groups attached to the parent chain, focusing on their number and location.
Prefixes: Unveiling the Diversity of Alkyl Groups
In the world of chemistry, the ability to accurately name and describe organic compounds is crucial for precise communication and understanding. IUPAC nomenclature, the standardized language of chemistry, provides a systematic framework for naming these compounds. At the heart of IUPAC nomenclature lie prefixes, which play a vital role in describing alkyl groups – the molecular building blocks of organic molecules.
Alkyl Groups: The Basic Units of Hydrocarbons
Alkyl groups are hydrocarbon chains that don’t possess any functional groups. They form the backbone of many organic compounds and are named based on their carbon count. Prefixes are used to indicate the number of carbon atoms in the alkyl group. For instance, the prefix “methyl” represents a one-carbon alkyl group, while “ethyl” refers to a two-carbon alkyl group.
Attaching Alkyl Groups to the Parent Chain
In naming organic compounds, the longest carbon chain is identified as the parent chain. Alkyl groups are then attached to the parent chain as substituents – groups that modify the parent chain’s structure and properties. Prefixes are used to denote the position and number of alkyl groups attached to the parent chain.
For example, the compound “2-methylbutane” contains a four-carbon parent chain (butane) with a methyl group (one carbon) attached to the second carbon atom. The prefix “2-” indicates the carbon number to which the methyl group is attached.
Importance of Prefixes
Prefixes provide a clear and concise way to describe the structure of organic compounds. They allow chemists to convey complex information about the number and location of alkyl groups, even for large and complex molecules. This standardized nomenclature ensures consistent communication and helps avoid confusion in scientific discourse.
By understanding the role of prefixes in IUPAC nomenclature, we gain a deeper appreciation for the intricate language of chemistry. It empowers us to accurately describe and identify organic compounds, facilitating effective communication and collaboration in the field of chemistry.
Double and Triple Bonds: Exploring Unsaturation and Reactivity
In the realm of chemistry, unsaturation refers to the presence of double or triple bonds between atoms, creating areas of high electron density and reactivity. These unsaturated bonds play a pivotal role in determining the chemical and physical properties of molecules.
Double Bonds: Sources of Unsaturation
Double bonds, denoted by the suffix “-ene,” consist of two shared pairs of electrons between two carbon atoms. They introduce a level of unsaturation, as the carbon atoms involved cannot form additional single bonds with other atoms. Double bonds create a region of high electron density, making them susceptible to electrophilic attack. This reactivity makes double bonds key players in a wide range of chemical reactions.
Triple Bonds: Unsaturated and Highly Reactive
Triple bonds, represented by the suffix “-yne,” are formed when three pairs of electrons are shared between two carbon atoms. They exhibit even higher unsaturation than double bonds, as the carbon atoms involved cannot participate in any additional single or double bonds. Triple bonds are highly reactive and can undergo a variety of addition reactions, showcasing their electrophilic nature.
Unsaturated Molecules and Their Properties
Molecules containing double or triple bonds are referred to as unsaturated. These bonds introduce areas of unsaturation, which influence both the reactivity and physical properties of the molecules. Unsaturated molecules are typically more reactive than saturated molecules, as the high electron density in the unsaturated bonds makes them attractive to electrophiles. Additionally, unsaturated molecules tend to have lower boiling points than saturated molecules, as the weaker forces of attraction between the unsaturated molecules result in a lower energy required for vaporization.
Understanding Unsaturation: A Key to Chemical Reactivity
Grasping the concept of unsaturation is crucial for understanding the reactivity of molecules. Double and triple bonds, as sources of unsaturation, dictate the chemical behavior and reactivity of many organic compounds. By recognizing the presence of unsaturated bonds, chemists can predict and control the course of chemical reactions, paving the way for targeted synthesis and the development of novel materials.
Unraveling the Complexity of Branching: A Guide to Chemical Nomenclature
In the realm of chemistry, where language matters, IUPAC nomenclature stands as the universal code for naming organic compounds. This system brings order to the chaotic world of molecules, allowing chemists to communicate with precision, especially when dealing with complex structures like branched molecules.
Let’s embark on a storytelling journey to understand how IUPAC nomenclature tackles the intricacies of branching. Imagine a parent chain, the longest continuous carbon chain in the molecule, like the backbone of a tree. Branching occurs when additional alkyl groups or functional groups extend from this backbone, resembling branches reaching out from the trunk.
To describe these branches, chemists employ a set of rules. Prefixes like “methyl,” “ethyl,” and “propyl” specify the number of carbons in each branch. These prefixes are assigned in descending order, ensuring that the largest branch is recognized first.
Suffixes, like “-yl” and “-ene,” provide clues about the nature of the branch. “-yl” indicates an alkyl group, while “-ene” signifies the presence of a double bond. Notations like “2-methyl” or “3-ethyl” tell us that the branch is attached to the parent chain at that particular carbon position.
For instance, consider the molecule pentane:
CH3-CH2-CH2-CH2-CH3
It has no branches, making it an unbranched alkane. But what if we add a methyl group to the second carbon?
CH3-CH(CH3)-CH2-CH2-CH3
Now, we have a branched molecule. The methyl group is attached to the second carbon of the parent chain, so we name it “2-methylpentane.”
Branching can get even more complex, involving multiple branches and functional groups. But the principles remain the same:prefixes describe the size and nature of the branches, suffixes indicate their presence and type, and the parent chain serves as the foundation.
By unraveling the complexity of branching through IUPAC nomenclature, chemists effectively translate molecular structures into a language that facilitates communication, research, and innovation.