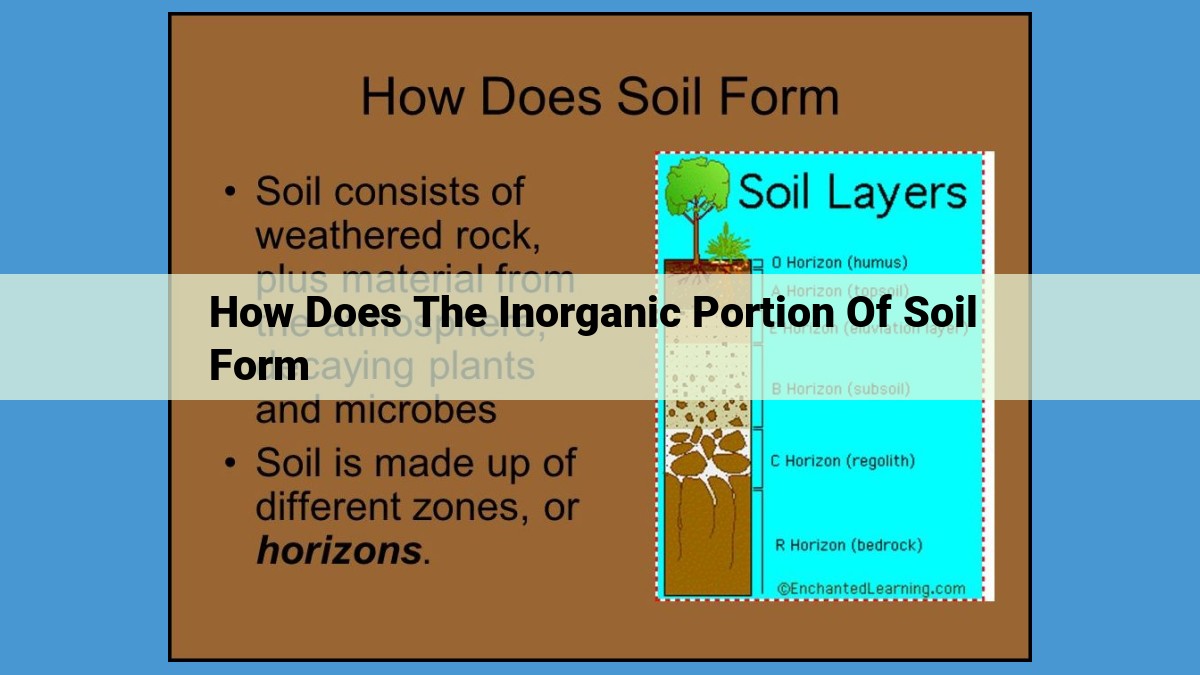
Inorganic soil formation begins with weathering, the physical and chemical breakdown of rocks and minerals. Organic matter is then converted to inorganic minerals through mineralization, enhancing soil health. Leaching, the downward movement of water, transports ions and salts, shaping soil chemistry. Acidification and alkalinization, the decrease and increase in soil pH, respectively, impact soil health and plant growth. Finally, oxidation-reduction reactions, the transfer of electrons, influence nutrient availability and soil chemistry.
Weathering: The Foundation of Soil Formation
In the realm of nature’s artistry, the unyielding power of weathering reigns supreme, transforming colossal rocks and dormant minerals into the fertile ground on which life thrives. Soil, the very essence of our planet’s ecosystems, owes its existence to this enigmatic process.
The Physical Dance of Breakdown
Weathering meticulously orchestrates the physical disintegration of rocks and minerals, reducing their bulky forms into minuscule fragments. Frost, a master craftsman, wields its icy grip, prying open fissures within rocks. As water seeps into these crevices and freezes, it expands with an inexorable force, causing the rock to shatter into smaller pieces. Heat, too, plays its role, alternating its embrace with cold. This thermal dance weakens the bonds within rocks, making them more vulnerable to further fragmentation.
The Chemical Symphony of Transformation
Beyond its physical prowess, weathering also wields the power of chemical alchemy. Water, imbued with a solvent’s touch, dissolves minerals, carrying them away as ions. Acids, both natural and anthropogenic, etch away at the rocky canvas, altering their composition. Oxygen, a ubiquitous presence in the atmosphere, forms alliances with minerals, giving rise to new compounds and reshaping the very nature of the soil.
The Significance of Weathering
The profound impact of weathering extends far beyond the mere production of soil. It shapes landscapes, carving out mountains and valleys. It nourishes ecosystems, releasing essential nutrients into the soil where plants can access them. Weathering is a testament to the interconnectedness of our planet, a tapestry woven by the intricate workings of nature’s forces.
Mineralization: The Alchemy of Soil Enrichment
In the realm of soil science, mineralization emerges as a vital process that transforms the dead and decaying remnants of living organisms into essential nutrients that nourish the very soil that sustains them. This intricate dance of decomposition and rebirth fuels the fertility and sustainability of our precious soils.
The journey of mineralization begins with the demise of plants, animals, and microorganisms. Their organic matter, composed of complex carbon compounds, embarks on a gradual transformation under the watchful eye of an army of microorganisms. These tiny architects of soil health wield their digestive enzymes, breaking down the organic matter into simpler components.
As this decomposition progresses, carbon dioxide is released into the atmosphere, while nitrogen and other essential nutrients are liberated. These nutrients, in turn, become bioavailable, meaning they can be easily absorbed by plants. This process not only enriches the soil with vital nourishment but also improves its structure and water-holding capacity.
The importance of mineralization cannot be overstated. It underpins the nutritional balance of soil ecosystems, supporting the thriving of diverse plant communities. Healthy soils, rich in organic matter and mineral nutrients, are the foundation for robust and sustainable agriculture.
By understanding the transformative power of mineralization, we can foster sustainable land management practices that nurture the health of our soils and safeguard the delicate balance of natural ecosystems.
Leaching: The Downward Movement of Nutrients
Imagine a subterranean river flowing through the depths of soil, carrying a vital cargo of dissolved ions and salts. This relentless downward journey, known as leaching, profoundly shapes the chemistry and fertility of the soil above.
Leaching occurs when water percolates through soil, carrying with it soluble minerals and organic matter. These substances, dissolved into the water, are transported downwards by the force of gravity. As they descend, they interact with soil particles, altering their composition and chemistry.
The rate of leaching is influenced by several factors, including the amount of rainfall, the soil’s texture and structure, and the presence of vegetation. Heavy rainfall can accelerate leaching, while dense soil and abundant vegetation can slow it down.
Leaching plays a crucial role in soil fertility. It removes excess salts, preventing them from accumulating to toxic levels. It also helps distribute nutrients evenly throughout the soil profile, ensuring that plants have access to essential elements.
However, leaching can also lead to the loss of valuable nutrients from the soil. This is especially concerning in agricultural areas, where excessive fertilization can result in nitrate leaching. Nitrates, when leached into groundwater, can contaminate drinking water sources and contribute to eutrophication in aquatic ecosystems.
Understanding the process of leaching is essential for sustainable soil management. By controlling factors such as rainfall and vegetation, farmers can minimize nutrient losses and protect the health of their soils. Proper irrigation techniques, crop rotations, and the use of cover crops can all help to reduce leaching and maintain soil fertility for future generations.
Acidification: Unraveling the Consequences on Soil and Ecosystems
Soil, the foundation of terrestrial life, is a dynamic and intricate system that sustains countless organisms and supports plant growth. However, certain processes can disrupt the delicate balance of soil, leading to detrimental effects on soil health and ecosystems. One such process is acidification, a phenomenon characterized by a decrease in soil pH due to natural or human activities. Understanding the causes and consequences of soil acidification is crucial for preserving the health of our planet’s soils.
Causes of Soil Acidification
Soil acidification occurs when the concentration of hydrogen ions (H+) in the soil increases. This can result from natural processes such as the decomposition of organic matter, which releases weak acids. However, human activities are major contributors to soil acidification. These activities include:
- Acid rain: Emissions of pollutants like sulfur dioxide (SO2) and nitrogen oxides (NOx) can form sulfuric and nitric acids in the atmosphere. When these acids return to the ground through rain, they lower soil pH.
- Fertilizers: Excessive use of nitrogen-based fertilizers can lead to nitrification, a process that releases hydrogen ions as a byproduct.
- Irrigation: Irrigation water often contains dissolved salts. When the water evaporates, these salts accumulate in the soil, increasing soil acidity.
Consequences of Soil Acidification
Acidification disrupts the chemical equilibrium of soil, affecting its physical, chemical, and biological properties. Acidified soils have reduced nutrient availability, particularly for essential elements like calcium, magnesium, and potassium. This can lead to stunted plant growth and poor crop yields. Acidification also damages soil structure, making it more compact and less permeable. This can hinder root development and reduce water infiltration, leading to drought stress in plants.
Acidification can also have profound effects on soil microorganisms, the unseen heroes responsible for nutrient cycling and soil fertility. Many beneficial bacteria and fungi are sensitive to low pH levels, and their decline can disrupt soil ecosystem functioning. Acidification can also mobilize toxic metals, such as aluminum, which can be harmful to plant roots and soil organisms.
Impacts on Ecosystems
The consequences of soil acidification extend beyond individual plants and soil health. Acidification can lead to:
- Reduced biodiversity: Acidified soils support fewer plant species, reducing the diversity of ecosystems and their ability to provide ecosystem services such as pollination and carbon sequestration.
- Water quality degradation: Acidified soils can leach nutrients and toxic metals into waterways, harming aquatic ecosystems.
- Climate change: Acidification can release carbon dioxide from soil, contributing to global climate change.
Mitigating Soil Acidification
To mitigate soil acidification and protect the health of our soils, we must:
- Reduce emissions: Implement strategies to reduce sulfur dioxide and nitrogen oxide emissions from industries and vehicles.
- Use fertilizers responsibly: Follow recommended application rates for nitrogen-based fertilizers and consider using organic alternatives.
- Improve irrigation practices: Use water sources with low salt content and avoid overwatering.
- Apply lime: Liming is a common practice to neutralize soil acidity. Acidified soils can be treated with agricultural lime, which raises soil pH.
Soil acidification is a serious threat to soil health and ecosystems. By understanding the causes and consequences of acidification, and implementing responsible land management practices, we can protect and preserve this vital resource for future generations.
Alkalinization: The Rise in Soil pH and Its Impact on Fertility
Soil, the lifeblood of our planet, is a complex and dynamic ecosystem that undergoes constant chemical transformations. One such transformation is alkalinization, a gradual increase in soil pH due to the accumulation of basic salts. Understanding the causes and consequences of alkalinization is crucial for maintaining soil health and the well-being of our agricultural systems.
Causes of Alkalinization:
Alkalinization can occur naturally or be accelerated by human activities. Natural processes include the weathering of minerals, such as limestone and dolomite, and the evaporation of water in arid regions. Human activities, such as applying alkaline fertilizers or irrigating with high-pH water, can also contribute to alkalinization.
Consequences of Alkalinization:
The increase in soil pH has several consequences for soil fertility and plant growth:
- Nutrient Availability: Alkalinization reduces the solubility of certain nutrients, such as iron, manganese, and zinc, making them less available to plants. This can lead to nutrient deficiencies and stunted plant growth.
- Soil Structure: High pH soils can have poor soil structure, which affects root penetration and water infiltration. This can increase erosion and reduce soil productivity.
- Beneficial Microorganisms: Some beneficial soil microorganisms, such as nitrogen-fixing bacteria, are sensitive to alkaline conditions and may decline in population. This can impact soil fertility and plant growth.
- Plant Sensitivity: Different plant species have varying tolerance to alkaline soils. Some plants, such as alfalfa and soybeans, are more tolerant, while others, such as blueberries and rhododendrons, are more sensitive.
Managing Alkalinization:
Managing alkalinization is essential for preserving soil health and agricultural productivity. Several strategies can be employed to mitigate the effects of high soil pH:
- Amendments: Adding acidic amendments, such as sulfur or gypsum, can lower soil pH. However, it’s important to follow soil testing recommendations to avoid over-acidification.
- Cropping Practices: Growing crops that are tolerant to alkaline conditions can help maintain soil productivity. Cover crops can also help improve soil structure and reduce erosion.
- Irrigation Practices: Using acidic irrigation water or blending high-pH water with more neutral sources can help reduce soil alkalinization.
- Liming Mitigation: In areas where liming is necessary to improve soil acidity, applying lime judiciously and according to soil test recommendations can minimize the risk of excessive alkalinization.
By understanding the causes and consequences of alkalinization and implementing effective management strategies, we can protect soil health and ensure the sustainability of our agricultural systems.
Oxidation-Reduction Reactions: Shaping the Chemistry of Our Soil
When we think of soil, we often picture a static entity, a foundation for plants and the basis of our agricultural systems. However, beneath the surface, a vibrant and active world unfolds, where chemical reactions play a crucial role in shaping the health and fertility of our soils. Among these reactions, oxidation-reduction (redox) reactions are the unsung heroes, silently orchestrating the availability of nutrients, the health of soil ecosystems, and even the fate of pollutants in our environment.
The Dance of Electrons: Oxidation and Reduction
Redox reactions involve the transfer of electrons between substances. When one substance loses electrons, it undergoes oxidation, while the substance receiving those electrons undergoes reduction. In soil, this dance of electrons is essential for many vital processes.
Nutrient Availability: A Redox Symphony
Redox reactions directly influence the availability of nutrients for plants. Nitrogen, a key nutrient for plant growth, is released into the soil through the oxidation of organic matter. Iron, another essential nutrient, becomes more soluble and accessible to plants under reducing conditions.
Soil Health and Microbial Thriving
The balance of redox reactions in soil shapes the microbial community that thrives within it. Aerobic microbes, such as bacteria and fungi, thrive in oxygen-rich conditions and facilitate the decomposition of organic matter. Anaerobic microbes, on the other hand, flourish in environments with limited oxygen and play a crucial role in the formation of certain gases, such as methane and hydrogen sulfide.
Environmental Impacts: Redox Reactions in Action
Redox reactions also have profound implications for environmental health. The reduction of toxic metals, such as mercury and lead, decreases their mobility and toxicity in soil. Conversely, the oxidation of sulfides can release harmful sulfur compounds into the atmosphere. Understanding these redox processes is essential for mitigating the negative impacts of pollution on our soils and ecosystems.
Oxidation-reduction reactions are the invisible conductors of soil chemistry, shaping the availability of nutrients, the health of soil ecosystems, and the fate of pollutants in our environment. By understanding these reactions, we gain valuable insights into the dynamic and complex world beneath our feet, empowering us to steward our soils and ensure their continued health for generations to come.