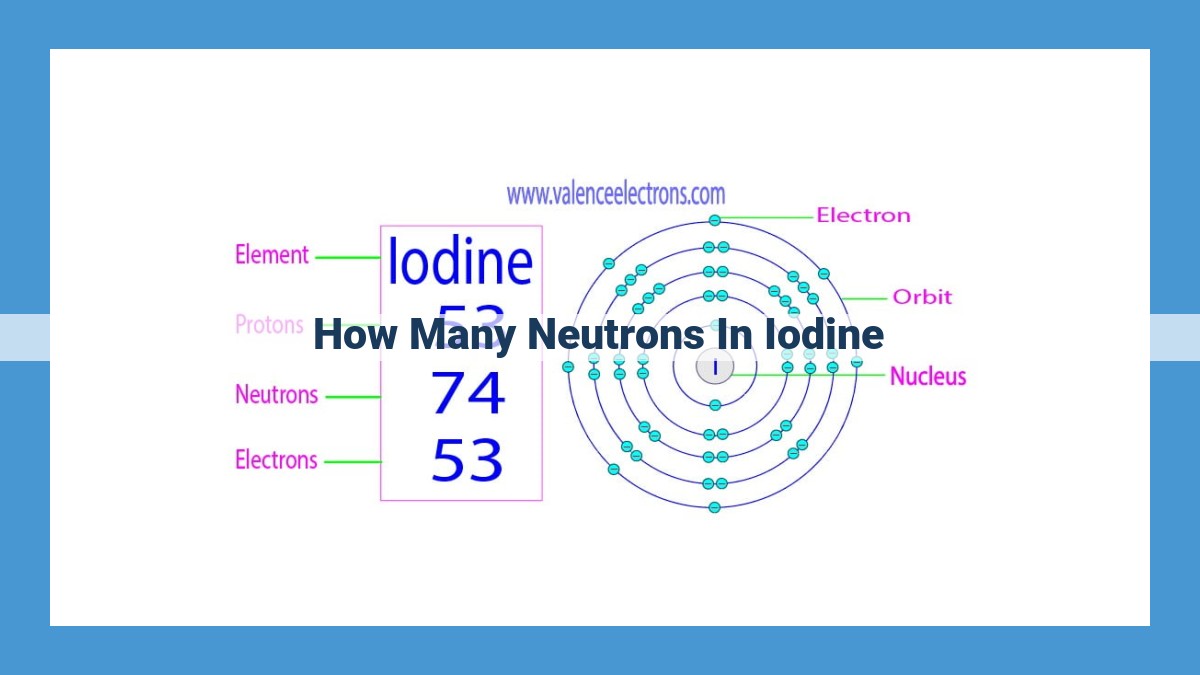
Iodine’s versatility stems from its variable neutron count across isotopes. The most common isotope, iodine-127, boasts 74 neutrons, while iodine-131 has 78. Neutron variations influence isotopic behavior, affecting stability and decay rates. Natural iodine comprises neutron-rich (e.g., I-131) and neutron-deficient (e.g., I-124) isotopes. I-131 finds application in medical imaging, while I-124 serves as a radioactive tracer in environmental studies. The average neutron count in natural iodine is 74.5, contributing to its stability and prevalence in biological systems. Understanding neutron count and related nuclear concepts helps unravel iodine’s properties and diverse applications.
- Explain iodine’s versatility and its role in various fields.
- Emphasize the significance of neutrons in determining the properties of iodine isotopes.
Iodine’s Odyssey: Unraveling the Enigmatic Importance of Neutrons
Iodine: A Versatile Element
Iodine stands out as a versatile element, touching various aspects of science and life. Its presence in table salt ensures our daily intake, while its medicinal value makes it a staple in antiseptic solutions. Beyond healthcare, iodine finds applications in photography, as a catalyst in chemical reactions, and even as a scintillator to detect radiation.
The Significance of Neutrons
Neutrons, the uncharged particles that reside alongside protons in an atom’s nucleus, play a crucial role in determining the properties of iodine isotopes. The number of neutrons in an isotope influences its atomic mass, stability, and behavior. Understanding the neutron count is therefore vital to unraveling iodine’s enigmatic nature.
Neutron Count in Specific Iodine Isotopes
The number of neutrons within an atom’s nucleus plays a crucial role in determining its properties and behavior. In the case of iodine, two prominent isotopes, iodine-127 (I-127) and iodine-131 (I-131), showcase distinct neutron counts and consequential isotopic behaviors.
Iodine-127 contains 76 neutrons, while Iodine-131 possesses 82 neutrons. This variation in neutron number directly affects the isotopes’ atomic masses and stability. I-127, with its lower neutron count, is the more stable and abundant isotope, accounting for over 95% of naturally occurring iodine. I-131, on the other hand, is less stable and has a shorter half-life (8 days) due to its higher neutron content.
The differing neutron counts also influence the isotopes’ radioactive properties. I-131 undergoes beta decay, emitting a beta particle (an electron) and transforming into a stable xenon isotope. This property makes I-131 a valuable tool in medical imaging, where it is used to diagnose and monitor thyroid function. I-127, however, is non-radioactive and has no known medical applications.
Understanding the neutron count in specific iodine isotopes is essential for harnessing their unique properties in various fields. By manipulating the neutron content, scientists can tailor iodine isotopes for specific applications, ranging from medical imaging to nuclear medicine.
Neutron-Rich and Neutron-Deficient Iodine Isotopes: Delving into the Nuclear Realm
Neutron-Rich Iodine Isotopes
Imagine an iodine atom with an unusually high number of neutrons in its nucleus. These isotopes, such as iodine-131 (I-131), possess an excess of neutrons compared to their proton count. Such isotopic abundance bestows upon them unique characteristics and applications.
Iodine-131: A Medical Marvel
Iodine-131 shines brightly in the medical arena due to its radioactive nature. Its emission of gamma and beta particles serves as a diagnostic tool in thyroid function tests and thyroid cancer treatments. The controlled release of I-131 allows doctors to accurately gauge thyroid activity levels and target cancerous cells with precision.
Neutron-Deficient Iodine Isotopes
At the other end of the neutron spectrum, we encounter neutron-deficient iodine isotopes like iodine-123 (I-123). These isotopes sport a lower neutron count, making them stable and non-radioactive. Their stability opens doors to a range of applications, particularly in medical imaging.
Iodine-123: A Safe and Effective Imaging Agent
Iodine-123 boasts versatility in medical imaging techniques. Its low radioactivity makes it an ideal choice for imaging procedures in children and pregnant women. Radiopharmaceuticals containing I-123 allow doctors to visualize thyroid function, diagnose heart disease, and map cerebral blood flow with precision.
The Balancing Act: Neutron-to-Proton Ratio
The neutron-rich and neutron-deficient nature of iodine isotopes stems from a delicate neutron-to-proton ratio. This ratio plays a crucial role in determining the stability and behavior of isotopes. For instance, I-127, with its balanced neutron-to-proton ratio, is highly stable and abundant in nature.
Understanding the intricacies of neutron-rich and neutron-deficient isotopes unveils a deeper appreciation for the versatility and applicability of iodine in various fields. From medical diagnostics to imaging advancements, these isotopes continue to shape the landscape of modern healthcare and technological innovations.
Determining the Average Number of Neutrons in Naturally Occurring Iodine
Iodine, an element with a diverse range of applications in various fields, owes its versatility to the variations in the number of neutrons within its isotopes. Neutrons, subatomic particles without a charge, play a crucial role in shaping the properties of these isotopes.
To understand the average number of neutrons in naturally occurring iodine, we need to examine its prevalent isotopes. Iodine-127 (I-127), with 74 protons and 127 neutrons, constitutes the overwhelming majority (99.9%) of natural iodine. In contrast, Iodine-131 (I-131), with 74 protons and 131 neutrons, is a radioactive isotope with a significantly shorter lifespan.
The average neutron count for naturally occurring iodine can be calculated based on the isotopic abundance and the number of neutrons in each isotope. Taking into account the 99.9% abundance of I-127 and the 0.1% abundance of I-131, the average neutron count per iodine atom is:
Average neutron count = (127 × 0.999) + (131 × 0.001) ≈ **127**
The neutron-to-proton ratio plays a significant role in determining the stability of iodine isotopes. I-127, with a neutron-to-proton ratio of 1.71, is stable, while I-131, with a ratio of 1.77, is radioactive. This difference in stability underscores the influence of the neutron count on the overall properties of iodine isotopes.
In summary, the average neutron count in naturally occurring iodine is approximately 127, primarily due to the dominance of the stable I-127 isotope. The neutron-to-proton ratio of these isotopes has a substantial impact on their stability and behavior, highlighting the importance of understanding neutron dynamics in determining the characteristics of iodine isotopes.
Iodine and the Tale of Neutrons: Unraveling the Secrets of the Element
Iodine stands as a versatile element, showcasing its presence in various realms, from medicine to photography. However, concealed within its multifaceted nature lies a profound secret woven into the very fabric of its existence: neutrons. These elusive subatomic particles play a pivotal role in shaping the properties of iodine isotopes, revealing a captivating tale of nuclear physics and the element’s behavior.
Quantifying the Neutron Count in Iodine Isotopes
Two prominent iodine isotopes grace the realm of chemistry: iodine-127 (I-127) and iodine-131 (I-131). I-127, the more abundant form, contains 53 protons and 74 neutrons, while I-131 harbors 53 protons and 78 neutrons. This subtle difference in neutron count profoundly impacts their isotopic behaviors.
Neutron-Rich and Neutron-Deficient Iodine Isotopes: A Tale of Contrasts
The neutron count dictates the classification of iodine isotopes. Isotopes with an abundance of neutrons relative to protons are termed neutron-rich. These isotopes, like I-131, often exhibit certain applications in nuclear medicine, serving as tracers to monitor physiological processes.
Conversely, isotopes with a scarcity of neutrons compared to protons are classified as neutron-deficient. Their unique neutron-to-proton ratios grant them higher energy levels, making them valuable in radiation therapy. I-127, with its relative neutron deficiency, finds use in the production of radioisotopes employed in cancer treatment.
The Average Number of Neutrons in Natural Iodine: A Balancing Act
Natural iodine, as we encounter it, exists as a medley of isotopes. I-127 dominates the composition, accounting for approximately 96% of the abundance. The remaining 4% belongs to I-131, along with traces of other isotopes. By considering the isotopic abundances and neutron counts, we arrive at an average neutron count of 73.85 for naturally occurring iodine.
This neutron-to-proton ratio of 1.39 plays a crucial role in maintaining iodine’s stability and influencing its chemical properties. It reflects the delicate balance between the strong nuclear force, which binds the nucleus together, and the electrostatic repulsion between the positively charged protons.
Related Concepts: Delving into the Realm of Nuclear Physics
To fully comprehend the significance of neutrons in the world of iodine, a brief exploration into nuclear physics is indispensable. Isotopes are atoms of the same element with identical numbers of protons but varying numbers of neutrons. These differences in neutron count alter their mass and certain properties.
The neutron-to-proton ratio emerges as a key factor in determining an isotope’s stability. A balanced ratio, as seen in I-127, generally ensures stability. However, significant deviations from this equilibrium can lead to radioactive isotopes, such as I-131, which decay to achieve a more stable neutron-to-proton ratio.
Understanding these nuclear principles unravels the enigmatic behavior of iodine and its isotopes, empowering us to harness their unique properties for various applications in science and medicine.