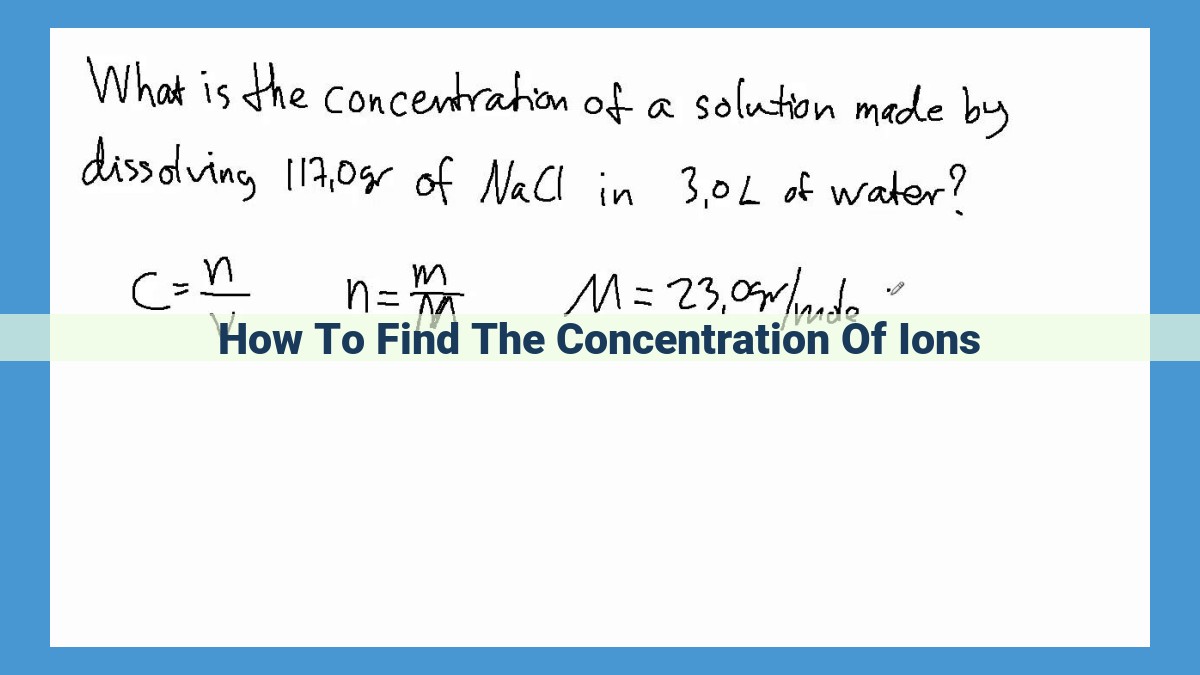
To determine the concentration of ions in a solution, various methods can be employed. One common approach involves calculating the concentration based on molarity (M), molality (m), or normality (N). Additionally, the equivalence point in a titration, where the moles of reactants are equal, can be utilized to determine ion concentrations through volumetric analysis techniques. Gravimetric analysis, ion chromatography, and inductively coupled plasma mass spectrometry (ICP-MS) are specialized methods used to quantify specific ions in complex matrices. These techniques play a critical role in various fields, including environmental chemistry, analytical chemistry, and medicine, where precise measurement of ion concentrations is essential for understanding and manipulating chemical systems.
Understanding Ion Concentration: A Comprehensive Guide
What is Ion Concentration?
In the world of chemistry, we encounter various substances that dissolve into ions, carrying an electrical charge. The concentration of ions refers to the amount of these charged particles dissolved in a solution. It’s measured as the number of ions per unit volume or the number of moles of ions per liter.
Importance of Ion Concentration
Ion concentration plays a vital role in numerous fields:
- Biology: Ion balance is crucial for cell function, nerve impulses, and maintaining healthy body fluids.
- Environmental Chemistry: Ion concentration in water bodies affects aquatic life and water quality.
- Medicine: Ion concentration is measured to diagnose and treat medical conditions, such as electrolyte imbalances.
- Industry: Ion concentration is monitored in industrial processes, including food production, pharmaceuticals, and chemical manufacturing.
Explaining Ion Concentration Units
When measuring ion concentration, different units are used to express the amount of ions present:
- Molarity (M): The number of moles of ions per liter of solution.
- Molality (m): The number of moles of ions per kilogram of solvent.
- Normality (N): The number of equivalent weights of ions per liter of solution.
Calculating Ion Concentration
Determining the ion concentration in a solution involves straightforward calculations using the appropriate unit:
- Molarity: Concentration (M) = Moles of ions / Volume of solution (L)
- Molality: Concentration (m) = Moles of ions / Mass of solvent (kg)
- Normality: Concentration (N) = Equivalent weight of ions / Volume of solution (L)
Equivalence Point and Titration
Titration is a technique used to determine the concentration of a known ion by reacting it with a solution of a known ion concentration. The point at which the ions are completely neutralized is called the equivalence point. This point allows us to calculate the unknown ion concentration.
Volumetric Analysis Techniques
Volumetric analysis methods, such as titration, are used to measure ion concentration by determining the volume of a known solution required to react completely with the unknown solution. Other volumetric methods include Mohr titration, argentometric titration, and complexometric titration.
Gravimetric Analysis
Gravimetric analysis determines the concentration of an ion by measuring the mass of the precipitate formed when it reacts with a suitable reagent. This method provides accurate results but is generally less versatile than volumetric methods.
Ion Chromatography
Ion chromatography is a powerful technique for separating and measuring ion concentrations. It uses ion exchange resins to separate ions based on their charge, allowing for precise quantification.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
ICP-MS is a highly sensitive analytical technique used to determine the concentration of metal ions in a solution. It utilizes a plasma to ionize the atoms of the metal and then measures the abundance of ions.
Related Concepts
Understanding ion concentration involves familiarity with related concepts:
- Concentration: The amount of a substance present in a unit of space or volume.
- Mass: The quantity of matter in an object.
- Moles: A unit of measurement for the amount of a substance.
- Volume: The amount of space occupied by a substance.
Applications in Various Fields
Ion concentration measurement has wide-ranging applications:
- Environmental Chemistry: Monitoring water quality, detecting pollutants, and understanding environmental processes.
- Analytical Chemistry: Determining the composition of substances, identifying unknown compounds, and verifying product quality.
- Medicine: Diagnosing and monitoring medical conditions, developing new therapies, and optimizing drug delivery.
Different Units of Ion Concentration: Understanding the Language of Chemistry
In the world of chemistry, ions – charged atoms or molecules – play a crucial role in various processes, and their concentration is a key factor that chemists need to measure and understand. To accurately describe and compare ion concentrations, several units have been developed, each serving a specific purpose and providing different insights.
Molarity (M):
Molarity is the most commonly used unit for expressing ion concentration. It measures the number of moles of solute (ions) per liter of solution. One mole of a substance represents a specific amount, defined as Avogadro’s number (approximately 6.022 x 10^23 particles). This unit is particularly useful for stoichiometric calculations, where the balanced chemical equation provides the mole ratios between reactants and products.
Molality (m):
Unlike molarity, which is influenced by temperature due to changes in solution volume, molality measures the number of moles of solute per kilogram of solvent. This unit is particularly beneficial when dealing with solutions that undergo significant temperature changes, ensuring that the concentration remains unaffected by thermal expansion or contraction.
Normality (N):
Normality is a unit that focuses on the equivalence of acid or base solutions. It measures the number of equivalents of solute per liter of solution. One equivalent is defined as the amount of substance that reacts with or is equivalent to one mole of hydrogen ions (H+). Normality is primarily used in acid-base titrations and neutralization reactions.
Choosing the Right Unit:
The choice of which unit to use depends on the specific application. Molarity is generally preferred for stoichiometric calculations and when dealing with dilute solutions. Molality is best suited for situations where temperature changes are a concern, such as in equilibrium studies. Normality is primarily used in acid-base chemistry.
Practical Applications:
Understanding the different units of ion concentration is essential in various fields. In environmental chemistry, measuring the concentration of ions in water samples can provide insights into water quality and pollution levels. In analytical chemistry, ion concentration measurements are used in titration analysis and gravimetric analysis, determining the composition and purity of substances. In medicine, ion concentration plays a vital role in maintaining electrolyte balance and diagnosing certain diseases.
Calculating the Concentration of Ions in Solutions
In the realm of chemistry, ion concentration plays a pivotal role, dictating the behavior and properties of various solutions. Understanding how to calculate ion concentration is essential for diverse fields, ranging from environmental monitoring to pharmaceutical research.
Units of Ion Concentration
The concentration of ions can be expressed using several units:
- Molarity (M): The number of moles of ions per liter of solution.
- Molality (m): The number of moles of ions per kilogram of solvent.
- Normality (N): The number of equivalents of ions per liter of solution.
Calculating Ion Concentration
To calculate the concentration of ions in a solution, we need to know:
- The total number of moles of ions in the solution.
- The volume of the solution in liters (for molarity).
- The mass of the solvent in kilograms (for molality).
- The equivalent weight of the ion (for normality).
Once we have these values, we can use the following formulas:
Molarity (M) = Moles of ions / Volume of solution (L)
Molality (m) = Moles of ions / Mass of solvent (kg)
Normality (N) = Equivalents of ions / Volume of solution (L)
Example Calculation
Let’s calculate the molarity of a solution that contains 0.1 moles of sodium ions (Na+) in 500 milliliters of solution.
Molarity (M) = 0.1 moles / 0.5 L = 0.2 M
Understanding how to calculate ion concentration is crucial for interpreting chemical data and predicting the behavior of solutions. By employing the appropriate units and formulas, we can accurately determine the concentration of ions in a variety of applications.
Equivalence Point: The Crucial Crossroads in Titration
In the realm of chemistry, the equivalence point stands as a pivotal moment in the process of titration, a technique used to precisely determine the concentration of an unknown solution. Imagine a chemist carefully adding a known solution with a known concentration, known as the titrant, to a sample of the unknown solution, called the analyte.
As the titrant flows into the analyte, a chemical reaction takes place, consuming the ions of interest. The progress of this reaction can be monitored using various indicators, which undergo a distinct color change when the equivalence point is reached. This point marks the moment when the moles of titrant added are chemically equivalent to the moles of analyte present, resulting in a complete reaction.
The equivalence point is a crucial landmark in titration because it allows us to calculate the unknown concentration of the analyte. By measuring the volume of titrant required to reach equivalence, we can determine the exact number of moles of titrant used. Using stoichiometry, we can then calculate the moles of analyte present in the sample, and from there, its concentration.
Knowing the equivalence point is essential for accurate and precise ion concentration measurements. It provides a clear endpoint for the titration and ensures that the reaction has gone to completion. Without determining the equivalence point accurately, the calculated ion concentration may be incorrect.
Therefore, the equivalence point serves as a guiding star in titration, leading us to reliable and meaningful ion concentration measurements. By carefully observing the color change of the indicator, chemists can pinpoint this critical point and unlock the secrets of unknown solutions, paving the way for further analysis and applications.
Volumetric Analysis Techniques: The Art of Precise Measurement
In the realm of chemistry, accurately determining the concentration of ions is crucial. One of the most widely used methods for this purpose is volumetric analysis, a technique that relies on precise volume measurements to determine the concentration of an unknown analyte. Volumetric analysis has a rich history, with its roots in the early days of quantitative chemistry.
One of the most fundamental principles of volumetric analysis is titration, a process that involves adding a known volume of a standard solution of known concentration to the analyte solution until an equivalence point is reached. At the equivalence point, the moles of the standard solution added are chemically equivalent to the moles of the analyte present in the sample.
Volumetric analysis techniques offer several advantages. Firstly, they are relatively simple to perform and require minimal specialized equipment. Secondly, they are precise, providing accurate and reproducible results. Finally, they are versatile and can be used to determine the concentration of a wide range of ions.
There are various types of volumetric analysis techniques, each tailored to specific applications. For instance, acid-base titrations are used to determine the concentration of acids or bases, while precipitation titrations are used to determine the concentration of ions that form insoluble precipitates. Complexometric titrations are used to determine the concentration of metal ions by forming soluble complexes with them.
The versatility of volumetric analysis techniques extends beyond the laboratory. They are widely used in various industries, including medicine, environmental monitoring, and food processing, where accurate ion concentration measurements are critical for quality control, safety, and compliance.
In summary, volumetric analysis techniques are a powerful tool for determining ion concentrations. They are precise, versatile, and relatively simple to perform, making them essential in various fields that demand accurate chemical measurements.
Gravimetric Analysis: Unveiling Ion Concentrations with Precision
In the realm of analytical chemistry, gravimetric analysis emerges as a foundational technique for determining the concentration of ions in solutions. This meticulous process relies on the principle of mass equivalence: the mass of a solid precipitate formed during a chemical reaction is directly proportional to the mass of the analyte ion present.
How does gravimetric analysis work?
- A known mass of the sample solution is taken and treated with a precipitating reagent, which selectively reacts with the target analyte ion to form an insoluble precipitate.
- The precipitate is then carefully filtered, washed, and dried to remove any impurities.
- The dried precipitate is weighed, and its mass is recorded.
Calculating ion concentration:
The mass of the precipitate is used to calculate the mass of the analyte ion using the stoichiometry of the precipitation reaction. The ion concentration can then be determined by dividing the mass of the analyte ion by the volume of the sample solution, expressed in units such as parts per million (ppm) or milligrams per liter (mg/L).
Advantages of gravimetric analysis:
- High accuracy and precision
- Simple and straightforward procedure
- Minimal need for specialized equipment
- Applicable to a wide range of ions
Applications of gravimetric analysis:
Gravimetric analysis finds numerous applications in various fields, including:
- Environmental chemistry: Determining the concentration of heavy metals in water and soil samples
- Analytical chemistry: Quantifying the purity of chemicals
- Biochemistry: Measuring the concentration of proteins and nucleic acids
- Pharmaceutical industry: Ensuring the accuracy of drug formulations
Gravimetric analysis remains an indispensable technique for determining ion concentrations with high precision. Its straightforward approach and wide applicability make it a valuable tool for scientists and analysts alike, providing reliable insights into the composition of solutions, ensuring accuracy in various scientific disciplines.
Ion Chromatography: A Powerful Tool for Separating and Quantifying Ions
Understanding ion concentration is crucial in various fields, including environmental monitoring, analytical chemistry, and medicine. It’s essential in determining the composition and properties of solutions and plays a vital role in controlling chemical reactions and biological processes.
Different Units of Ion Concentration
Ion concentration can be expressed in several units:
- Molarity (M): The number of moles of ions per liter of solution (e.g., 0.1 M NaCl solution contains 0.1 moles of NaCl ions per liter).
- Molality (m): The number of moles of ions per kilogram of solvent (e.g., 0.1 m NaCl solution contains 0.1 moles of NaCl ions per kilogram of water).
- Normality (N): The number of equivalent weights of ions per liter of solution (e.g., 0.1 N HCl solution contains 0.1 equivalent weights of H+ ions per liter).
Concentration of Ions in Solutions
Calculating the concentration of ions in solutions involves converting between different units using stoichiometry and solution volume. Accurately determining ion concentration is essential for various analytical methods, such as titration and volumetric analysis.
Ion Chromatography
Principles of Ion Chromatography
Ion chromatography is a powerful analytical technique used to separate and quantify ions in complex mixtures. It employs an ion exchange resin to selectively retain ions based on their charge and affinity for the resin. A mobile phase, containing an eluent, flows through the resin, carrying the ions along with it. Different ions elute from the column at different times, allowing for their separation and detection.
Applications of Ion Chromatography
Ion chromatography has numerous applications, including:
- Environmental analysis: Determining ion concentrations in water, soil, and air samples for pollution monitoring.
- Industrial analysis: Analyzing ion content in products and manufacturing processes to ensure quality control.
- Food analysis: Measuring ion concentrations in food and beverages for safety and nutritional assessment.
- Clinical chemistry: Detecting and quantifying ions in biological samples for medical diagnosis and monitoring.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): A Powerful Tool for Metal Ion Analysis
Unveiling the Secrets of Metal Ions with ICP-MS
In the realm of analytical chemistry, Inductively Coupled Plasma Mass Spectrometry (ICP-MS) stands tall as a cutting-edge technique that empowers scientists to unravel the mysteries of metal ion concentrations with remarkable precision and accuracy. This sophisticated instrument harnesses the power of inductively coupled plasma (ICP), a superheated ionized gas, to excite and fragment metal ions in a sample. By measuring the mass-to-charge ratio of these fragments, ICP-MS provides a detailed fingerprint of metal ion concentrations, revealing their presence and abundance.
Applications: A Multifaceted Analytical Tool
ICP-MS has become an indispensable tool in a wide array of fields, including:
- Environmental monitoring: Detecting and quantifying metal ion contamination in air, water, and soil samples to ensure environmental health and regulatory compliance.
- Forensic science: Identifying trace metal ion evidence in criminal investigations, such as gunshot residue or blood samples.
- Geochemistry: Analyzing metal ion concentrations in geological samples to understand the origin, evolution, and composition of the Earth’s crust.
- Medical diagnostics: Measuring metal ion levels in biological fluids to diagnose and monitor diseases, such as heavy metal poisoning or iron deficiency.
The Magic of ICP-MS: A Closer Look
The ICP-MS technique involves introducing the sample into an argon plasma, which heats it to extremely high temperatures (up to 10,000 Kelvin). This intense heat causes the metal ions in the sample to become excited and fragmented. The resulting ions are then introduced into a mass spectrometer, which separates them based on their mass-to-charge ratio. By analyzing the abundance of each ion, scientists can determine the concentration of the corresponding metal ion in the sample.
Benefits: Speed, Sensitivity, and Versatility
ICP-MS offers several notable advantages:
- Speed: Rapid analysis times, allowing for quick and efficient sample processing.
- Sensitivity: Detects even trace levels of metal ions, down to parts per trillion or quadrillion.
- Versatility: Analyzes a wide range of metals, from alkali metals to heavy metals.
- Robustness: Tolerates complex sample matrices, making it suitable for various sample types.
Inductively Coupled Plasma Mass Spectrometry (ICP-MS) is a transformative technology that has revolutionized the analysis of metal ion concentrations. Through its unparalleled capabilities in detecting and quantifying metal ions with precision and sensitivity, ICP-MS has become an indispensable tool in a diverse array of fields, from environmental protection to medical diagnostics. As technology continues to advance, we can expect even more innovative applications of ICP-MS in the years to come, unlocking new insights into the world of metal ion chemistry.
Related Concepts in Ion Concentration
- Discuss related concepts such as concentration, mass, moles, and volume, and their importance in ion concentration calculations.
Related Concepts in Ion Concentration
Understanding ion concentration is essential in various scientific disciplines, but it’s equally crucial to grasp related concepts like concentration, mass, moles, and volume. These concepts are interwoven in ion concentration calculations and play a vital role in understanding the chemistry of solutions.
Concentration refers to the amount of a substance present in a given volume or mass of a solution. Mass represents the total amount of a substance present, while moles indicate the number of atomic or molecular units present. Volume is the measure of space occupied.
When dealing with solutions, the unit of concentration used depends on the specific application. Molarity (M) expresses the number of moles of solute per liter of solution. Molality (m) indicates the number of moles of solute per kilogram of solvent. Normality (N) represents the number of equivalent weights of solute per liter of solution. Each of these units has its specific advantages and is chosen based on the requirements of the particular situation.
Understanding these related concepts is essential for accurate calculations involving ion concentration. For example, in a titration, the equivalence point is reached when the number of moles of titrant added is stoichiometrically equivalent to the number of moles of analyte present. Understanding the concepts of moles, concentration, and stoichiometry enables accurate determination of the analyte’s concentration.
In summary, ion concentration is a cornerstone of chemistry, and a thorough understanding of related concepts like concentration, mass, moles, and volume is crucial for accurate calculations and comprehension of chemical reactions. By delving into these concepts, we can unlock the secrets of ion concentration and its profound implications in scientific fields.
Applications of Ion Concentration Measurement
Environmental Chemistry:
- Monitoring water quality for pollutants like heavy metals and nitrates
- Studying soil composition to assess fertility and contamination
- Investigating acid rain and its effects on ecosystems
Analytical Chemistry:
- Identifying and quantifying ions in food and pharmaceutical samples
- Determining trace elements in environmental samples
- Verifying the purity of chemical products
Medicine:
- Measuring electrolyte levels in blood to diagnose and treat electrolyte imbalances
- Monitoring drug concentrations in serum for effective and safe treatment_
- Analyzing urine samples to diagnose kidney and metabolic disorders
Other Applications:
- Industrial processes: Controlling ion concentrations in chemical reactions
- Food production: Monitoring fermentation and preservation processes
- Clinical research: Investigating the role of ions in biological systems_
Ion concentration measurement plays a vital role in diverse fields. It helps us ensure environmental health, analyze chemical substances, manage medical conditions, and advance scientific research. By understanding the importance and applications of ion concentration measurement, we can appreciate its impact on our lives and the environment.