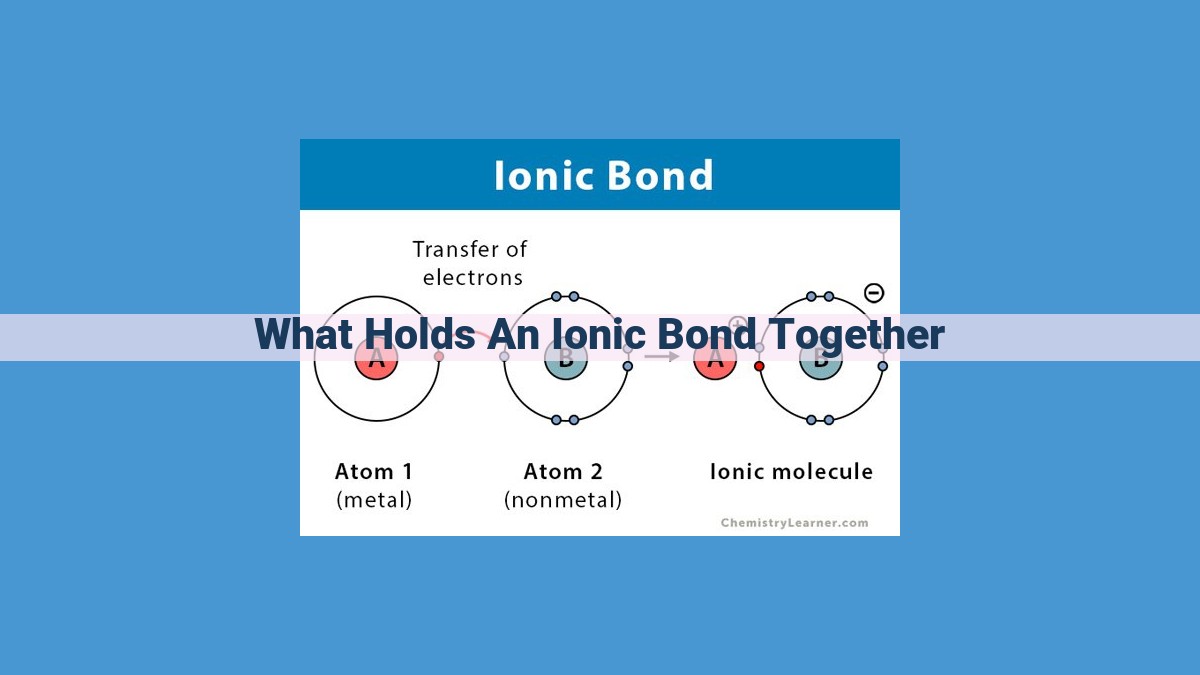
Ionic bonds are held together by the electrostatic force of attraction between positively charged metal ions and negatively charged nonmetal ions. The opposite charges interact through Coulomb’s Law, resulting in a strong electrostatic attraction. This attraction is further enhanced by the formation of a crystal lattice structure, where ions are arranged in a regular pattern. The tightly packed crystal lattice and the high lattice energy contribute to the high melting and boiling points of ionic compounds. In polar solvents like water, ionic compounds dissolve because the solvent molecules surround and separate the ions, weakening the electrostatic attraction and allowing the ions to disperse.
Electrostatic Force of Attraction: The Invisible Force Shaping Our World
Prepare to embark on a captivating journey into the realm of Electrostatic Force of Attraction, the silent orchestrator behind the formation and properties of countless substances around us. Join us as we unravel the secrets of this intriguing force and witness its profound impact on our world.
Defining the Electrostatic Force and Coulomb’s Law
Imagine a world without attraction. Scattered objects would float aimlessly, and life as we know it would cease to exist. Electrostatic Force of Attraction is the invisible bond that holds atoms and molecules together, ensuring the stability of matter. According to Coulomb’s Law, two electrically charged objects exert a force on each other that is proportional to the product of their charges and inversely proportional to the square of the distance between them.
Electric Charge and the Formation of Ionic Bonds
At the heart of Electrostatic Force of Attraction lies electric charge. Matter comprises tiny building blocks called atoms, which contain positively charged protons and negatively charged electrons. When electrons are transferred between atoms, they gain or lose an electrical charge, creating ions. Positive ions (cations) are attracted to negative ions (anions), forming ionic bonds.
Electric Potential: A Measure of Charge Concentration
Electric potential is a crucial concept that describes the tendency of a charged particle to move from one point to another. It measures the potential energy per unit charge at a given location. High electric potential indicates a higher concentration of charge, leading to stronger electrostatic forces.
These fundamental principles lay the foundation for our understanding of Electrostatic Force of Attraction, a force that governs the structure and behavior of a vast array of substances, from simple salts to complex minerals. Prepare to delve deeper into the fascinating world of ionic compounds and explore the remarkable properties that arise from this invisible force.
The Unbreakable Bond: Formation of Ionic Compounds
In the realm of chemistry, where atoms mingle and molecules dance, ionic bonding stands out as a tale of attraction and electron exchange. Imagine two oppositely charged ions, one positive and the other negative, like magnets drawn to each other.
This irresistible force, the electrostatic attraction, is the driving force behind the formation of ionic compounds. These compounds are the guardians of our everyday world, hidden within the walls of our homes and the screens of our devices.
The dance of ions begins with the transfer of electrons from one atom to another. Metals, eager to shed their excess electrons, play the role of the donor, while nonmetals, hungry for electrons, become the recipients. This act of giving and receiving creates a charged ion, an atom that has gained or lost electrons.
The resulting ions are then locked in an embrace, their opposite charges holding them together. The positive ion, or cation, orbits the negative ion, or anion. This intimate dance forms the foundation of the ionic bond, an unyielding force that binds these ions together.
Strong Electrostatic Attraction: The Unbreakable Bonds of Ionic Compounds
Ionic bonds, the unyielding bonds that hold ionic compounds together, are a testament to the immense power of electrostatic attraction. In this realm of electrostatic dance, ions, with their opposite charges, sway effortlessly towards one another, forming an indissoluble union.
Ionic Bond Strength and Lattice Energy
The strength of an ionic bond, measured by its lattice energy, is directly proportional to the charges of the ions involved and inversely proportional to the distance between them. The larger the charges and the smaller the distance, the stronger the bond.
Crystal Lattice Structures: Order from Chaos
Ionic compounds don’t merely aggregate in a haphazard manner. Instead, they arrange themselves in highly ordered structures known as crystal lattices. These lattices consist of repeating units called unit cells, which define the compound’s shape and properties. The study of these structures is known as crystallography.
Crystal Defects: The Imperfect Harmony
Even in the seemingly flawless realm of ionic crystals, imperfections arise. These crystal defects disrupt the perfect lattice arrangement, influencing the compound’s properties. They can affect its color, electrical conductivity, and reactivity. Understanding and controlling these defects is crucial in the design and development of advanced materials.
Ionic Compounds: Mighty Giants with High Melting and Boiling Points
High Melting and Boiling Points
Ionic compounds, like brave knights in shining armor, stand tall and strong, refusing to yield to the forces of melting and boiling. This exceptional resilience stems from the formidable electrostatic attraction that binds their positively and negatively charged ions together, forming a tightly packed crystal lattice.
Imagine a castle composed of countless tiny building blocks, each interlocking seamlessly with its neighbors. In ionic compounds, these building blocks are the ions, and the castle they form is the crystal lattice. The stronger the electrostatic attraction between the ions, the more tightly they grip each other, creating a more robust fortress.
This remarkable bond strength, known as lattice energy, is the secret behind the high melting and boiling points of ionic compounds. To melt or boil an ionic compound, we must overcome the immense electrostatic attraction holding the crystal lattice together. It’s like trying to break through the walls of a fortress defended by an army of invisible warriors.
Water Solubility:
- Explain the process of hydration of ions.
- Describe the role of polar solvents in dissolving ionic compounds.
- Discuss the dissociation of ionic compounds in water.
Water Solubility of Ionic Compounds
In the realm of chemistry, ionic compounds stand apart as unique substances characterized by their strong electrostatic attraction. This magnetic bond between oppositely charged ions shapes their behavior in many ways, including their solubility in water.
Hydration of Ions
When an ionic compound encounters water, a remarkable transformation occurs. Water molecules, with their polar* character, surround the **ions like tiny magnets, forming a protective shell called the hydration sphere. This hydration process weakens the electrostatic attraction between ions, making it easier for them to separate and dissolve into the water.
Polar Solvents and Dissolution
The ability of water to dissolve ionic compounds stems from its polar nature. The oxygen atom in water carries a partial negative charge, while the hydrogen atoms possess a partial positive charge. These charged regions attract the oppositely charged ions, pulling them apart and facilitating their dissolution.
Dissociation in Water
Upon dissolving in water, ionic compounds undergo a complete dissociation into individual ions. This process is driven by the solvating power of water, which weakens the electrostatic forces holding the ions together. The ions then become surrounded by water molecules, forming hydrated ions that can move freely in solution.
In essence, the water solubility of ionic compounds is a testament to the intricate interplay between the electrostatic attraction between ions and the polar nature of water. This unique characteristic grants ionic compounds their ability to dissolve and contribute to various chemical reactions in aqueous environments.