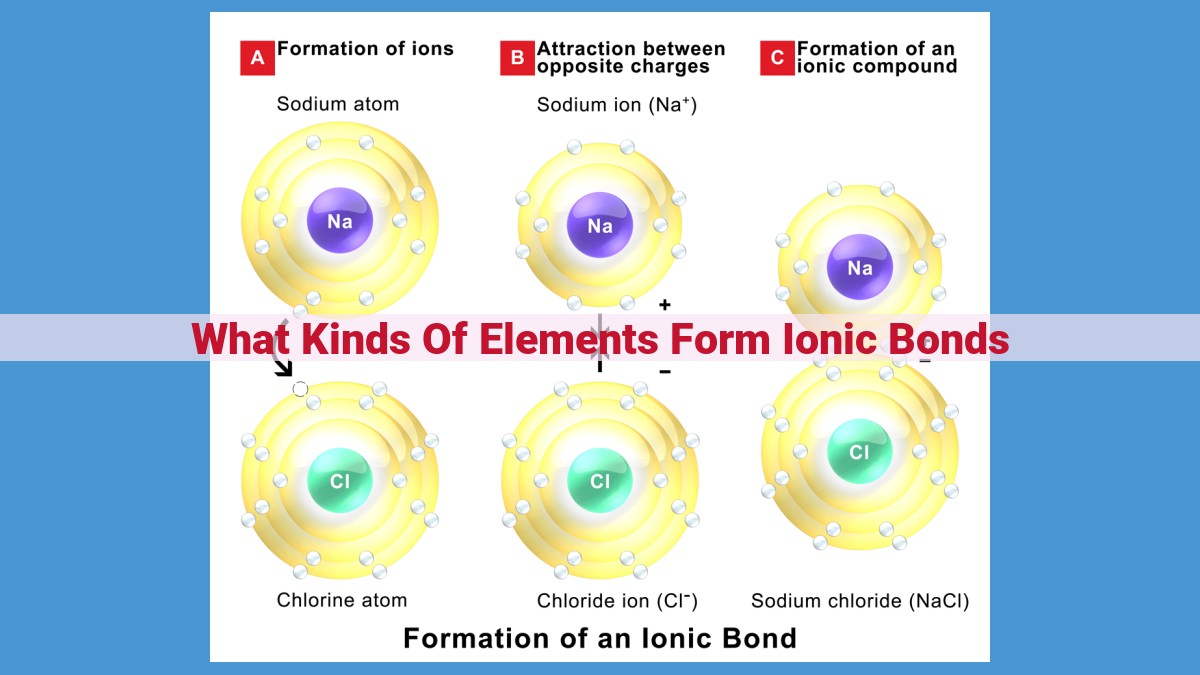
Ionic bonds form when elements with significantly different electronegativities interact. Metals, with low electronegativities, readily lose electrons, forming cations. Nonmetals, with high electronegativities, attract electrons, forming anions. The electrostatic attraction between these oppositely charged ions results in ionic bonding. Elements prone to ionic bond formation include highly electropositive metals (e.g., Na, K) and highly electronegative nonmetals (e.g., Cl, F).
Ions, the Building Blocks of Matter:
In the vast tapestry of chemistry, ions play a pivotal role as fundamental particles that determine the properties of matter. Ions are atoms or molecules that have lost or gained electrons, giving them an electrical imbalance. This imbalance results in a net charge, either positive or negative.
Cations and Anions: Two Sides of the Same Coin:
Based on their charge, ions are classified into two main types: cations and anions. Cations are ions with a positive charge, while anions are ions with a negative charge. The loss of electrons creates cations, and the gain of electrons creates anions.
Understanding the Dance of Ionic Bonding:
When a metal atom loses one or more electrons, it becomes a positively charged cation. These cations are attracted to negatively charged anions, which are formed when nonmetal atoms gain electrons. The electrostatic force between these oppositely charged ions gives rise to a powerful ionic bond.
Electronegativity: The Key to Bond Formation:
Electronegativity is a measure of an atom’s ability to attract electrons in a bond. Metals tend to have low electronegativity, while nonmetals have high electronegativity. This difference in electronegativity drives the formation of ionic bonds, as atoms strive to achieve stability by transferring electrons.
Valence Electrons and the Octet Rule:
The reactivity of atoms in ionic bond formation is largely determined by their valence electrons. These are the electrons in the outermost energy level of an atom. Atoms seek to attain a stable configuration by either losing or gaining electrons to achieve a full valence shell, typically eight electrons, known as the octet rule.
Identifying Ionic Bond-Forming Elements:
Elements that are most likely to participate in ionic bond formation are electropositive metals such as sodium and potassium, which have low electronegativity and readily lose electrons. On the other hand, electronegative nonmetals like chlorine and oxygen have a high affinity for electrons and readily accept them to form anions.
Understanding the concepts of ions and ionic bonding is crucial for unraveling the mysteries of chemical reactions and the properties of matter. By grasping these fundamental principles, we gain a deeper appreciation for the intricate interactions that shape our universe at the atomic level.
Ionic Bonding: The Dance of Atoms
In the realm of chemistry, atoms, the building blocks of all matter, engage in a fascinating dance called ionic bonding. This dance involves a transfer of electrons that creates ions, charged particles that hold powerful electrostatic attractions.
The Electron Shuffle: Electrons on the Move
Ionic bonding begins with an electron shuffle. Metals, with their outer electron orbits loosely held, graciously release these electrons to nonmetals, which have a strong affinity for them. This transfer transforms the metal atom into a cation, a positively charged ion, while the nonmetal becomes an anion, a negatively charged ion.
The Electrostatic Tango: Attraction Unfolds
The newly formed ions, with their opposite charges, experience an irresistible electrostatic attraction. Like a captivating waltz, they gracefully glide towards each other, forming an ionic bond. This bond is a result of the electrostatic forces between the positively charged cation and the negatively charged anion.
Unveiling the Characteristics of Ionic Bonding
Ionic bonding is a unique dance with distinctive characteristics:
- High Melting and Boiling Points: The strong electrostatic attractions between ions require a significant amount of energy to overcome, resulting in high melting and boiling points.
- Brittleness: Ionic compounds are often brittle due to the rigid structure formed by the oppositely charged ions.
- Solubility in Water: Ionic compounds readily dissolve in water because the polar water molecules can disrupt the electrostatic interactions between the ions.
Ionic bonding stands as a testament to the captivating interactions between atoms. Through the transfer of electrons and the irresistible electrostatic attraction that follows, ions find stability and form the building blocks of countless ionic compounds. Understanding ionic bonding is crucial for unraveling the mysteries of chemical structures and reactions, providing a glimpse into the vibrant dance of atoms.
Electronegativity: The Key to Ionic Bond Formation
In the world of chemistry, understanding the dance between atoms is crucial for grasping the formation of molecules and compounds. Electronegativity plays a central role in this dance, especially when it comes to the creation of ionic bonds.
Electronegativity measures an atom’s affinity for electrons. Metals tend to have low electronegativity values, meaning they willingly give up their electrons, while nonmetals have high electronegativity values, indicating a strong desire to attract electrons. When a metal atom encounters a nonmetal atom, a tug-of-war ensues for electrons.
Consider sodium, a metal with a low electronegativity (0.9), and chlorine, a nonmetal with a high electronegativity (3.0). As sodium’s lone valence electron is not held tightly, chlorine’s strong attraction for electrons pulls it away from sodium. This electron transfer transforms sodium into a positively charged cation, Na⁺, and chlorine into a negatively charged anion, Cl⁻.
The resulting electrostatic attraction between the oppositely charged ions creates an ionic bond, forming the ionic compound sodium chloride (NaCl). The electronegativity difference between sodium and chlorine, 2.1, is substantial enough to drive the complete transfer of electrons, leading to the formation of stable ions.
In essence, electronegativity dictates the tendency of atoms to lose or gain electrons, determining whether they will form ionic bonds. The greater the electronegativity difference between two atoms, the stronger the ionic bond they will form. This concept becomes critically important in predicting the formation of ionic compounds, paving the way for a deeper understanding of chemical reactions and the properties of matter.
Valence Electrons: The Key Players in Ionic Bonding
In the fascinating world of chemistry, atoms are the fundamental building blocks. Each atom has its own unique set of valence electrons—electrons that reside in the outermost energy level. These valence electrons determine how an atom interacts with other atoms, forming the chemical bonds that hold molecules together.
The Reactivity Factor
The number of valence electrons an atom possesses influences its chemical reactivity. Atoms with a few valence electrons tend to be highly reactive, eager to gain or lose electrons to achieve stability. On the other hand, atoms with a full valence shell—eight valence electrons—are more stable and less reactive.
The Octet Rule: A Quest for Stability
Atoms have a preference for a full valence shell, a phenomenon known as the octet rule. This rule states that atoms strive to gain, lose, or share electrons until they achieve a stable, eight-valence-electron configuration. The octet rule drives many chemical reactions, including the formation of ionic bonds.
Ionic Bonding: A Balancing Act
When an atom loses or gains electrons, it acquires an ionic charge. Ions are charged particles: atoms that have lost or gained electrons, such as cations (positively charged ions) and anions (negatively charged ions).
Ionic bonding occurs when atoms transfer electrons to achieve a stable, full valence shell. In this process, one atom donates electrons, becoming a positively charged cation, while another atom accepts those electrons, becoming a negatively charged anion. The electrostatic attraction between the oppositely charged ions holds the ionic compound together.
Elements that Form Ionic Bonds: A Chemical Alliance
In the captivating realm of chemistry, ions, electrically charged atoms, take center stage. They emerge when electrons, the tiny particles that dance around atomic nuclei, decide to embark on adventures beyond their usual confines. This transfer of electrons sets the stage for ionic bonding, an electrostatic dance between cations (positively charged ions) and anions (negatively charged ions).
The key to understanding which elements are most likely to form ionic bonds lies in their electronegativity. This concept, which measures an atom’s attraction for electrons, plays a pivotal role in determining the nature of chemical bonds. Metals, adorned with few valence electrons, are quick to shed their electronic companions, becoming electropositive. On the other end of the spectrum, nonmetals, with their electron-hungry nature, act as electronegative partners, eager to acquire electrons.
When an electropositive metal encounters an electronegative nonmetal, a thrilling exchange takes place. The metal, with its excess electrons, generously transfers them to the nonmetal, creating a positively charged cation and a negatively charged anion. This electrostatic attraction, like a magnetic force between two opposite poles, binds the ions together, forming an ionic bond.
Prominent examples of ionic compounds illustrate the power of this bond. Sodium chloride (NaCl), the common salt we sprinkle on our meals, is a prime example. Sodium, a highly electropositive metal, donates an electron to chlorine, an electronegative nonmetal, resulting in the familiar NaCl formula. Another classic example is potassium fluoride (KF), where potassium’s willingness to part with an electron complements fluorine’s strong desire for electrons.
In the grand scheme of chemical bonding, ionic bonding plays a pivotal role in shaping the properties of many substances. These compounds, often crystalline solids, are characterized by their high melting and boiling points, reflecting the strong electrostatic forces holding them together. They are also good conductors of electricity when dissolved in water or melted, as the mobile ions facilitate the flow of electrical current.
Understanding ionic bonding is an essential step in comprehending the diverse world of chemical interactions. By delving into the electronegativity of elements and the resulting transfer of electrons, we uncover the secrets behind the formation of these fascinating compounds that enrich our understanding of matter and its transformations.