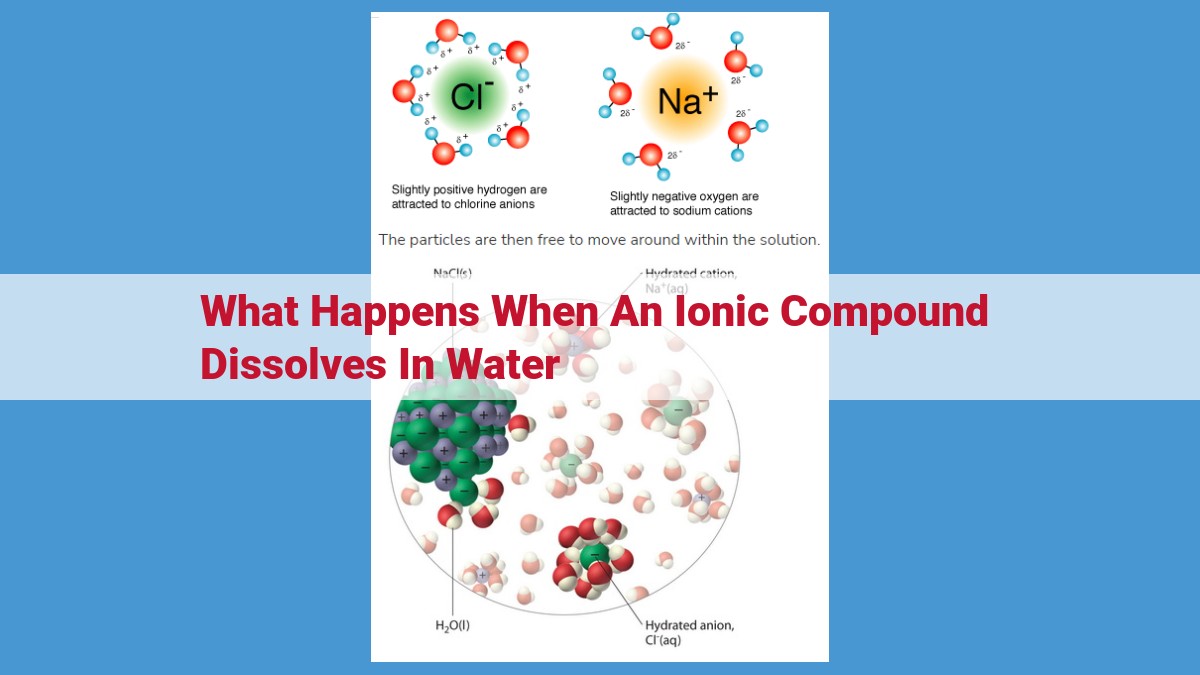
When an ionic compound dissolves in water, it undergoes a process of ionization, where its constituent ions separate due to the attraction between polar water molecules and their charges. These ions then become hydrated, forming a water shell around them for stability. The interaction between solvent molecules and solute is known as solvation. The dissolved ions can conduct electricity, giving the solution conductivity. The process of dissolution can release or absorb heat, resulting in exothermic or endothermic solutions, respectively.
Ionization: The Breaking Up of Ions
In the realm of chemistry, the world of atoms and molecules teems with an unseen dance of charge. Within this dance, certain molecules, like water, possess a unique polarity. Their molecules are like tiny dipoles, with one end slightly positive and the other slightly negative. This polarity empowers water to interact with remarkable substances called ions.
Ions, the charged counterparts of atoms or molecules, are born when an atom or molecule gains or loses electrons. These charged particles carry an inherent attraction for water molecules. As water surrounds ions, the positive water end is drawn to negative ions, while the negative water end embraces positive ions. This attraction leads to a process called ionization, where ions are separated from one another due to the irresistible pull of water’s polarity.
Hydration: Ions Get a Watery Embrace
When ionic compounds dissolve in water, a fascinating phenomenon occurs known as hydration. Imagine you’re an ion, floating alone in a vast expanse of water. Suddenly, water molecules rush towards you, drawn by the irresistible attraction between their polar charges and your own.
As the water molecules envelop you, they form a protective shell around you, like a cozy embrace. This water shell, known as the hydration sphere or solvation shell, plays a crucial role in stabilizing ions in solution. It shields them from interacting with other ions, preventing precipitation or the formation of solid particles.
Hydration has a profound impact on the stability and behavior of ions. It influences their mobility, solubility, and reactivity. Ions with a larger hydration sphere are more stable and less reactive because the water molecules hinder their ability to interact with other species.
For instance, sodium ions (Na+) have a smaller hydration sphere compared to potassium ions (K+). This difference in hydration affects their solubility in water, with K+ being more soluble due to its larger hydration sphere.
Understanding hydration is essential in understanding the behavior of ionic compounds in solution. It’s a fundamental concept in chemistry, with applications in various fields, including biology, environmental science, and electrochemistry. So, next time you dissolve an ionic compound in water, remember the magical dance that takes place, where ions find solace and stability in their watery embrace.
Solvation: Beyond the Watery Embrace of Ions
In the realm of chemistry, the interactions between molecules and ions are a fascinating dance. Solvation, a broader concept than hydration, explores the captivating interplay between solvent molecules and solutes.
In the case of ionic compounds, the hydration of ions is a crucial aspect of their behavior in solution. Water molecules, with their polar nature, are drawn to the charged ions, forming a water “shell” around them. This shell stabilizes the ions, preventing them from recombining and ensuring their solubility.
But solvation extends beyond hydration. It encompasses the interaction of ions with any solvent, not just water. The solvent molecules surround and interact with the ions, forming a protective layer. This layer influences the stability, solubility, and reactivity of the ions in solution.
The nature of the solvent plays a significant role in solvation. Different solvents have varying polarities, which can affect their ability to solvate ions. For example, polar solvents like water can effectively solvate ions, while nonpolar solvents like hexane have a weaker solvating ability.
The size and charge of the ions also influence solvation. Smaller ions are more strongly solvated than larger ions, and ions with higher charges are more effectively solvated than those with lower charges.
Solvation is a fundamental concept in chemistry, as it affects the properties and behavior of ions in solution. By understanding solvation, we gain insights into the behavior of ionic compounds and the role of solvent molecules in shaping their interactions.
Conductivity: Solutions That Spark
When you dissolve an ionic compound in water, it breaks down into positively charged cations and negatively charged anions. These ions are free to move around the solution, which is why ionic solutions can conduct electricity.
The ability of a solution to conduct electricity is called its conductivity. Conductivity is measured in siemens per meter (S/m). The higher the conductivity, the more easily the solution can conduct electricity.
Several factors influence the conductivity of a solution, including:
The concentration of the solution: The more ions there are in a solution, the more easily it can conduct electricity. This is because there are more ions available to carry the electrical current.
The temperature of the solution: The conductivity of a solution increases with temperature. This is because the ions move faster at higher temperatures, which allows them to carry the electrical current more easily.
The type of ions in the solution: Different types of ions have different conductivities. For example, sodium ions (Na+) have a higher conductivity than calcium ions (Ca2+). This is because sodium ions are smaller and lighter than calcium ions, which allows them to move more easily through the solution.
The conductivity of a solution is an important property because it can be used to determine the concentration of the solution and to identify the type of ions present. Conductivity is also used to control the flow of electricity in electrical devices.
Heat of Solution: A Thermodynamic Twist
When ionic compounds dissolve in water, they undergo a fascinating dance of energy exchange known as the heat of solution. Dive into the world of thermodynamics and unravel the secrets behind this intriguing phenomenon.
As an ionic compound enters the aqueous realm, its ions break free from their electrostatic embrace. This process, called ionization, is driven by the irresistible attraction between polar water molecules and the charged ions. However, this liberation comes at a cost.
The ionization process requires energy to overcome the electrostatic forces holding the ions together. This energy is absorbed from the surroundings, leading to a drop in temperature and an endothermic solution. On the flip side, when ions dissolve without absorbing energy from the environment, the solution is exothermic, releasing heat as the ions mingle with water molecules.
The heat of solution is a crucial factor in various chemical processes. For instance, in the synthesis of sodium chloride (table salt), the exothermic dissolution of sodium and chlorine ions releases heat, driving the reaction to completion. In contrast, the endothermic dissolution of ammonium chloride in water absorbs heat, creating a cooling effect.
Understanding the heat of solution is not only intriguing but also essential in fields like chemistry, pharmacy, and environmental science. It helps predict the behavior of ionic compounds in aqueous solutions, optimizing chemical reactions, drug formulations, and even environmental remediation strategies.
Next time you dissolve an ionic compound in water, take a moment to ponder the heat exchange unfolding before your eyes. It’s a testament to the intricate ballet of energy that governs the molecular world. And remember, as you witness this dance of ions, keep in mind the concept of heat of solution—a thermodynamic twist that adds a fascinating layer to the chemistry of our world.