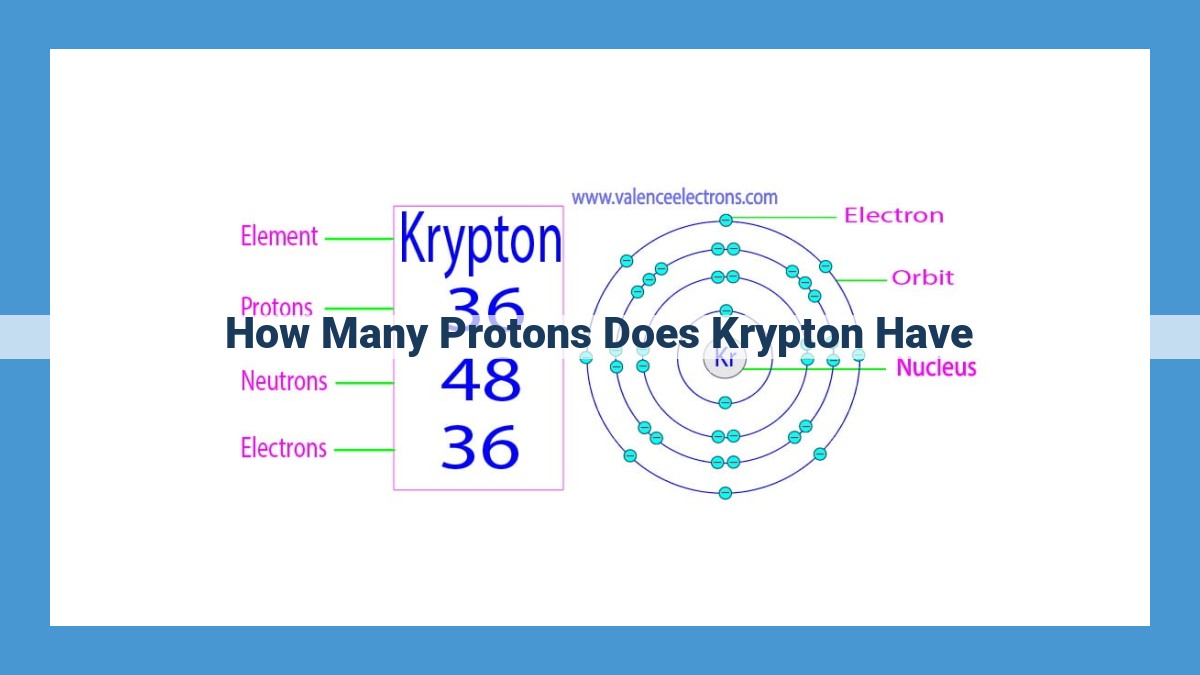
- An atom’s atomic number indicates the number of protons in its nucleus. For krypton, this number is 36, which means it has 36 protons.
**Unveiling the Secrets of Krypton: A Journey into Its Atomic Structure**
Picture this: Imagine the world of atoms – the minuscule building blocks that make up all matter. Atoms themselves are comprised of even smaller particles: protons, neutrons, and electrons.
Protons, the positively charged particles, reside in the atom’s core or nucleus. Neutrons, neutral in charge, accompany the protons in the nucleus, providing stability. Electrons, negatively charged particles, orbit the nucleus in energy levels called shells.
Each element is unique, defined by its specific number of protons. This defining characteristic is known as the atomic number. Krypton, with an atomic number of 36, boasts 36 protons in its nucleus, setting it apart from all other elements.
Krypton’s atomic structure is a testament to its individuality, a blueprint that dictates its chemical and physical properties. This journey into its atomic makeup is a captivating exploration into the very essence of matter.
Krypton’s Electron Configuration: Unraveling the Secrets of a Noble Gas
In the depths of the atomic realm, where particles dance with unseen energies, lies the fascinating world of electron configuration. This intricate arrangement of electrons around an atom’s nucleus holds the key to understanding the unique properties and behaviors of elements.
Electron Configuration and Orbital Notation: A Guiding Compass
Electrons reside in specific regions around the nucleus, forming layers known as energy levels or shells. Each shell can accommodate a certain number of electrons, with the first shell holding a maximum of two, the second eight, and so on. Within these shells, electrons occupy specific orbitals, which are regions of high electron probability.
Orbital notation provides a concise representation of an atom’s electron configuration. Each orbital is denoted by a letter (s, p, d, f) representing its shape, followed by a superscript indicating the number of electrons it contains. For instance, 1s² indicates two electrons occupying the first energy level, in the s orbital.
Krypton’s Electron Configuration: A Signature of Stability
Krypton, an element residing in Group 18 of the periodic table, holds a distinctive electron configuration: [Ar] 3d¹⁰ 4s² 4p⁶. This arrangement, with its outermost energy level filled with eight electrons, grants krypton the coveted title of a noble gas.
Chemical Properties Dictated by Configuration
The electron configuration of an element profoundly influences its chemical properties. The presence of a complete outermost energy level, as in krypton, renders the element chemically inert. Noble gases, like krypton, are reluctant to undergo reactions with other elements, hence their moniker as “inert gases.” This stability arises from the filled outermost energy level, which minimizes the atom’s tendency to gain or lose electrons.
The electron configuration of an element, like a detailed blueprint, provides a profound insight into its underlying structure and properties. By unraveling the secrets of krypton’s electron configuration, we gain a deeper appreciation for the enchanting tapestry of the atomic realm and the fundamental principles that govern the behavior of matter.
Krypton’s Place in the Periodic Table: A Tale of Noble Gases and Groupings
Within the vast expanse of the periodic table, krypton, our enigmatic element of focus, occupies a distinct position in Group 18 and Period 4. This strategic location grants it membership in the exclusive family of noble gases, a group of elements renowned for their chemical inertness.
The periodic table, a masterful tapestry of elements, organizes them based on their atomic number, which represents the number of protons in an atom’s nucleus. Krypton, with its atomic number of 36, boasts an equal number of electrons, the negatively charged particles that orbit the nucleus.
Element groupings, both vertically (groups) and horizontally (periods), reveal intriguing patterns and relationships. Elements within the same group, like krypton and the other noble gases, share similar electronic configurations, resulting in comparable chemical behaviors.
Krypton’s position in Group 18, also known as the noble gas group, is a testament to its inert nature. Noble gases possess stable electron configurations, with their outermost electron shells filled to capacity. This electronic stability renders them reluctant to participate in chemical reactions, hence their moniker “inert gases.”
Krypton’s placement in Period 4 signifies that it has four energy levels or “shells” surrounding its nucleus. The chemical properties of elements often exhibit periodic trends within a period. For example, krypton’s relatively low reactivity compared to elements in earlier periods reflects the increased shielding effect of its inner electrons.
In summary, krypton’s position in Group 18 and Period 4 of the periodic table provides valuable insights into its electronic configuration, chemical properties, and unique place among the elements.
Isotopes of Krypton: Unveiling the Diversity of an Inert Gas
What are Isotopes?
In the realm of chemistry, isotopes are distinct forms of the same element that share the same atomic number but differ in their neutron count. Every element’s atom comprises a nucleus filled with protons and neutrons and is surrounded by orbiting electrons. The number of protons defines the element’s atomic number, while the sum of protons and neutrons determines its atomic mass.
Krypton’s Isotopic Family
Krypton, a noble gas found in Group 18 of the periodic table, boasts a total of 32 known isotopes. These isotopes vary in their neutron count, ranging from 69 to 104. The most abundant isotope, constituting over 99% of naturally occurring krypton, is krypton-84.
Practical Applications of Krypton Isotopes
The different isotopes of krypton exhibit unique properties that lend themselves to a diverse range of applications.
- Krypton-84: This stable isotope finds use in gas lasers, such as the krypton fluoride laser employed in various scientific and industrial processes.
- Krypton-85: A radioactive isotope with a half-life of 10.76 years, krypton-85 is widely used in nuclear medicine. It serves as a tracer in lung scans, helping to detect and diagnose respiratory ailments.
- Krypton-86: Another radioactive isotope with a shorter half-life of 86 seconds, krypton-86 is instrumental in calibrating radiation detection equipment.
Unlocking the Secrets of Krypton
Delving into the world of krypton isotopes offers insights into the intriguing diversity present within a single element. Each isotope, with its unique neutron count, contributes to the rich tapestry of krypton’s properties, enabling its use in a plethora of practical applications. From lasers to medical diagnostics, the isotopes of krypton exemplify the subtle nuances that shape the world of chemical elements.
Chemical and Physical Properties of Krypton
Noble gases, including krypton, stand out in the realm of elements due to their extraordinary properties. They possess a remarkable stability and inertness, resulting from their complete electron shells. This unique characteristic makes them reluctant to participate in chemical reactions, giving them their designation as “noble.”
Krypton, positioned within Group 18 of the periodic table, exhibits properties that follow the periodic trends observed across the group. Its atomic number of 36 signifies the presence of 36 protons and an equal number of electrons, showcasing the trend of increasing atomic number within each period. Furthermore, krypton’s position in Period 4 indicates its four energy levels, aligning with the trend of increasing energy levels as one descends the periodic table.
One of the most striking physical properties of krypton is its colorless, odorless, and unreactive nature. This inertness stems from its stable electron configuration, with a full complement of eight electrons in its outermost energy level. This electron arrangement forms a protective shield around the atom, preventing it from interacting with other atoms or molecules.
In contrast to its chemical passiveness, krypton showcases unique physical properties that find practical applications. Its high density and low boiling point make it an ideal gas for filling incandescent and fluorescent lighting, where it enhances light output and extends bulb life. Additionally, krypton’s inertness and low thermal conductivity render it suitable for use in double-paned windows, aiding in insulation and energy conservation.
Krypton: The Noble Gas with Remarkable Applications
When discussing the element krypton, one cannot help but marvel at its unique properties and diverse applications that have revolutionized various fields. From illuminating our world to aiding in medical advancements, krypton’s versatility has made it an invaluable tool in modern society.
One of the most well-known uses of krypton is in fluorescent lighting. The vivid colors emitted by krypton-filled tubes have transformed the lighting industry, adorning streets, homes, and businesses alike. Additionally, krypton’s high-intensity light finds applications in specialized fields such as medical imaging and photography.
Beyond lighting, krypton plays a vital role in the development of lasers. Its high atomic number enhances the intensity and coherence of laser beams, making them indispensable in applications such as laser surgery, telecommunications, and scientific research.
In the medical realm, krypton makes significant contributions to nuclear medicine and radiation therapy. Radioactive isotopes of krypton are used in targeted therapies, enabling precise and minimally invasive treatments for various ailments.
For instance, Krypton-85 is employed in ventilational scans to assess lung function, while Krypton-79 serves as a tracer gas in perfusion studies, evaluating blood flow to organs. Additionally, krypton’s shielding properties make it suitable for use in radiotherapy to minimize radiation exposure to healthy tissues.
Krypton’s versatility extends to industrial applications as well. Its low reactivity and high thermal conductivity make it ideal for use in insulation and cooling systems. In aerospace, krypton is utilized in pressurized systems and as a propellant in ion engines.
In conclusion, krypton, once considered a mere curiosity, has emerged as a multifaceted element with remarkable applications. From enhancing our visual experiences to revolutionizing healthcare and advancing industries, krypton’s contributions are undeniable. Its versatility and unique properties continue to inspire innovation and progress, shaping our world in countless ways.